In this post I will delve into six key factors that impact your purification success in flash column chromatography.
Previously, I have discussed the use of TLC for solvent scouting and method development and optimization. I have have also talked about cartridge size, particle size, and surface area and their impact on flash purification. Here I integrate that information into the six factors below.
1. Solvent choice – impacts selectivity and mass transfer kinetics. Selectivity is a measure of the amount of separation between compounds. Mass transfer is the rate at which compounds adsorb and desorb from the silica stationary phase (partitioning rate in reversed-phase flash chromatography). The solvents you choose for your flash purification may provide good selectivity but may not be good “desorbers” showing up as broad or tailing chromatographic peaks. Choose your flash chromatography solvents based on how well they separate components on TLC, both in resolution and spot shape.
For example, if you get a good TLC separation with hexane/ethyl acetate but one or more of the spots tail, streak, or are broad/overlap as in a classic figure 8 separation, try replacing ethyl acetate with acetone at the same concentration. Why acetone? Because acetone and ethyl acetate share the same selectivity (VIa) and have similar strengths, 0.56 and 0.58, respectively, and therefore can typically be substituted for each other, figure 1.
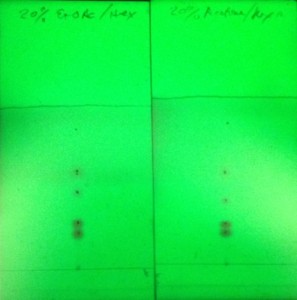
To show how acetone can improve flash purification, I separated the same 5-component mix using a gradient based on the above TLC data. The first purification used hexane/ethyl acetate (Figure 2) while the second purification used hexane/acetone (Figure 3).
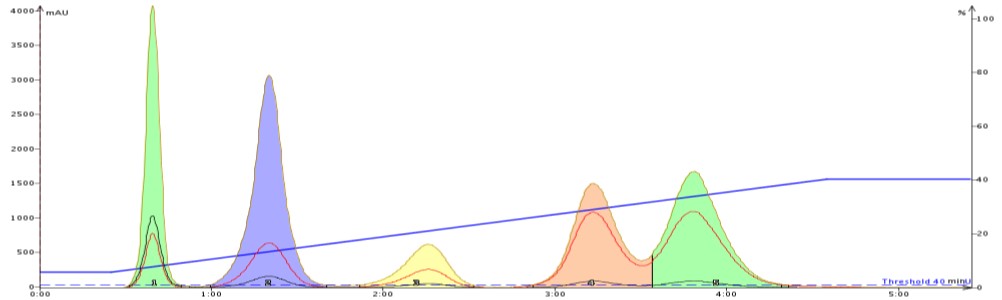
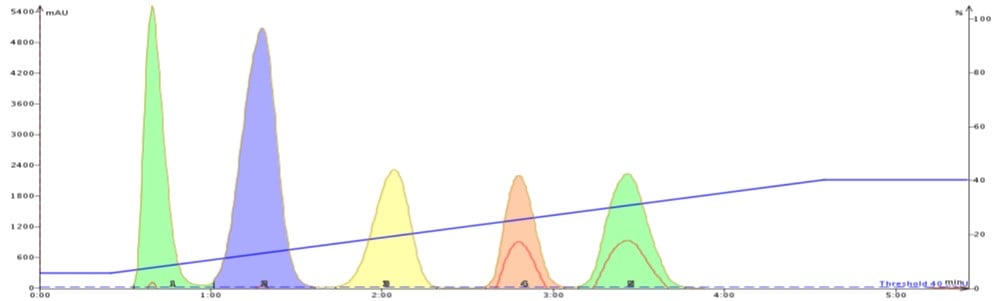
The improved adsorption/desorption kinetics from using acetone decreases both compound retention and peak width while increasing resolution. Clearly, selecting acetone over ethyl acetate as the strong solvent can speed up and improve your purification quality.
You may be concerned about using acetone because of its strong UV absorption between 215 nm and 330 nm. Well, modern automated flash systems such as the Biotage Isolera™ Spektra system eliminate this problem through use of a baseline subtraction algorithm which eliminates any baseline changes due to the solvents' UV absorption.
2. Particle size – impacts efficiency or plate count, which in turn impacts resolution. Efficiency is a measure of the amount of band broadening that occurs as a compound travels through a column or cartridge. Higher efficiency columns with smaller particles, provide narrower elution bands (peaks) and therefore increase separation between any pair or group of compounds, assuming you have selectivity! An increase in resolution increases the purity of any compound being purified – or – allows for an increase in loading.
3. Media surface area – directly impacts sample loading capacity; more surface area = more loading capacity. Simple. This is another variable within your control but may require some research as some flash cartridge suppliers do not publish this data. Cartridges containing high surface area media are available from here. These high surface area silica cartridges can enable you to step down a cartridge size to purify the same amount of material in less time with less solvent.
4. Media chemistry – impacts selectivity and mass transfer kinetics. Similar to solvent choice, which makes sense, as both the solvent and the media interact with the compounds moving through the column. Most chemists use silica but for some types of molecules, e.g. basic organic amines, this may not be the best choice. For more information about organic amine purification alternatives read my post on organic amine purification.
5. Dissolution solvent (sample solvent for injection)– impacts many areas in flash chromatography including efficiency, retention, selectivity, resolution. Too strong of a dissolution solvent and poor chromatography is the result. Methanol as a dissolution solvent in normal-phase flash, even in small amounts, can hinder your purification as it is preferentially adsorbed to silica compared to most organic compounds and interferes with their mass transfer kinetics. Choose a chromatographically weak solvent, maximize the concentration of your crude dissolved in it so you can minimize the volume injected. Another option is to dry load the sample by absorbing it onto silica or other inert sorbent (diatomaceous earth (Biotage® HM-N) or Florisil®); a topic for a future post!
6. Flow rate – impacts purification time, efficiency, and mass transfer kinetics. For every cartridge size and media particle size there is an optimal flow rate or linear velocity. Pump too slow or too fast and you will see a reduction on separation performance. However, for flash purification where time does matter, running at a slightly faster flow than optimal can speed your purification time without dramatically reducing purification quality, but there are limitations.
So, what is optimal? Smaller particle sizes allow the use of faster linear velocities and therefore faster flow rates. From what I have seen a linear velocity of between 3 cm/min (~0.5 mm/sec) and 12 cm/min (~2 mm/sec) provides the best separations with a 25 µm particle silica. For larger particles, say 50 µm, optimal linear velocity is lower, between 2 cm/min (~0.35 mm/sec) and 4.4 cm/min (0.7 mm/sec).
As you can see the listed variables are all within your control. So, know your purification goal and determine which media, elution solvents, dissolution solvent, and flow rates will help you achieve it.
I have also written about ways to create efficient purification methods to separate compounds in all types of mixtures.
Do you have a topic you would like addressed in the blog? If so, let me know.
Interested in learning more about flash chromatography? Download our whitepaper - Successful Flash Chromatography.

