Jan 19, 2023 6:27:00 PM
Is there an easy way to purify organic amines?
By Bob Bickler

If you synthesize organic amine compounds, especially heterocyclic, secondary, or tertiary amines, you likely have encountered problems with their chromatography using silica columns. With the amine groups being basic and silica being acidic, there is a natural attraction between the two. This sometimes strong attraction often requires the use of a competing amine in the solvent system. Modification of the mobile phase with the addition of a solvent like triethyl amine can provide a successful purification. Often times the use of an amine-modified stationary phase can provide the needed conditions to avoid the acid-base interaction that can interfere with a successful flash chromatography purification.
In this post I will discuss how amine-functionalized silica can simplify organic amine purification and your life (at least in the lab).
If you are making amine functionalized organic molecules and you find it challenging to purify them with normal-phase silica you are not alone. Many pharmaceutically active small molecules have these basic amine function groups, Figure 1. These basic amines are attracted to the acidic silanols on the silica and often require “aggressive” solvent mixtures such as methylene chloride/methanol/ammonia to overcome the acid-base attraction and elute the compound.
/Blogs%20ONLY/Trimipramine.jpg?width=283&height=186&name=Trimipramine.jpg) Figure 1. Structure of an organic amine (trimipramine) which can be challenging to purify on bare silica.
Figure 1. Structure of an organic amine (trimipramine) which can be challenging to purify on bare silica.
With both methanol and ammonia being very polar their ability to displace other polar compounds adsorbed on silica is quite high. Controlling the amount of methanol and ammonia so that compounds desorb fully but do not elute too quickly, though, is the problem most chemists face. Too little methanol or ammonia and the compounds do not elute or elute in very large volumes, too much and the compounds elute too quickly with little or no separation, Figure 2.
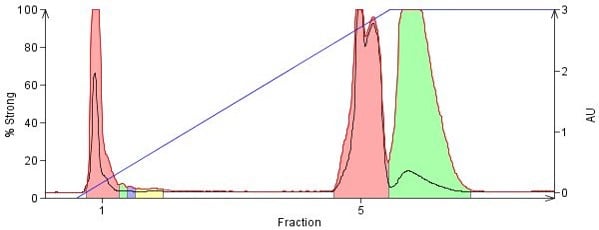
Figure 2. Normal-phase purification of phenylbenzylamine, promethazine, 4-dimethylamino antipyrine, and nifedipine on a 10 g silica column using a DCM/MeOH/NH3 solvent system. The last three compounds are polar and have strong attraction to the silica but with the addition of small amounts of methanolic ammonia, 4% in this case, they do elute but with little separation
In my experience with these types of compounds I have found amine-functionalized media work well. TLC plates with the same media are available for method development which usually results in efficient separations using hexane/ethyl acetate or ethyl acetate/isopropanol solvent systems – always without an added amine modifier.
In the Figure 3, the same 4-component organic amine mix is completely separated using an amine-functionalized flash column, Biotage® KP-NH, in a simple hexane/EtOAc gradient. With KP-NH, the amine-based stationary phase masks the silanols and minimizes the strong amine-silica interactions.
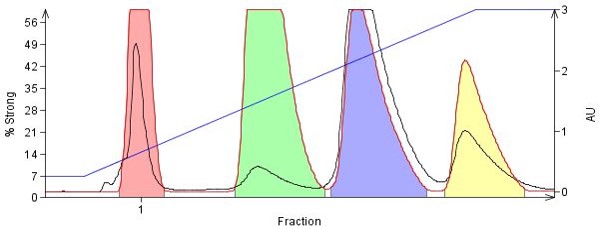
Figure 3. The same four amines from above were purified using an 11 g amine-functionalized silica cartridge (Biotage® KP-NH) using a hexane/EtOAc gradient. All compounds are fully separated yielding higher purity fractions.
So, if you are like me and want to simplify your purifications, try an amine-functionalized column the next time you need to purify organic amines.
If you want to learn more download our literature Inspiring Productivity in Modern Flash Chromatography.
Published: Jan 19, 2023 6:27:00 PM

