For many chemists using generic linear gradients and even gradients based on TLC the purification results often are not selective enough to separate all of the compounds in their mix. This is especially true if your target has a closely eluting impurity. One method used to try and increase resolution is the use of an isocratic hold or gradient pause during purification.
In this post I examine the use of the isocratic hold to determine how well it works and when/if it should be inserted into a gradient method.
During my career as a chromatographer I have met with many smart people who swear that if they watch their chromatography and pause the gradient or insert an isocratic hold when a compound begins to elute that the separation of that eluting compound improves. To me this seems illogical.
This is because at the moment that you see a peak being detected, the compound is already exiting the column, Figure 1. The stationary phase and its interaction with the solvents in the column have already performed the separation. Inserting an isocratic hold at this time is too late as it will only impact the solvent blend that has not yet entered the column. Any effect at all will only be observed on later eluting compounds. Thinking about this further, isn’t it logical to predict that at least one column volume of the isocratic solvent blend should be required before there can possibly be any impact from the isocratic hold?
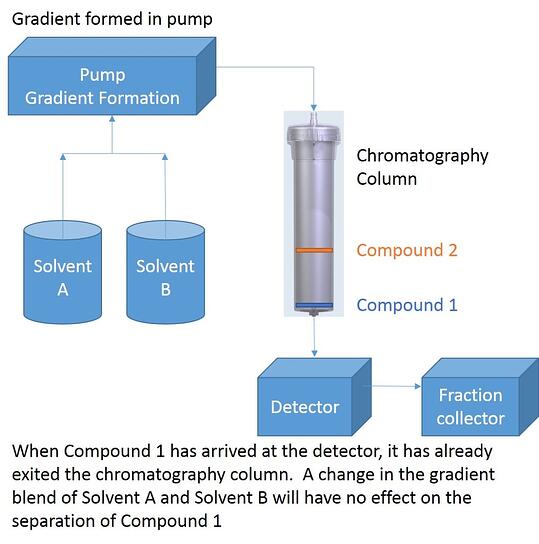
Figure 1. A flash chromatography system plumbing diagram showing the elution of two compounds. Compound 1 is leaving the cartridge and entering the detector so any gradient modification will not impact its separation from neighboring compounds.
In previous posts I have discussed ways to optimize flash purification, most often utilizing TLC. The use of an isocratic hold is something I recently experimented with as I wanted to find out if and when it is appropriate to apply a hold to a separation.
TLC
To start, I ran TLC of a 5-component mix in 20% ethyl acetate / 80% hexanes, calculated Rf values, and used them to build my linear gradient, Figure 2.
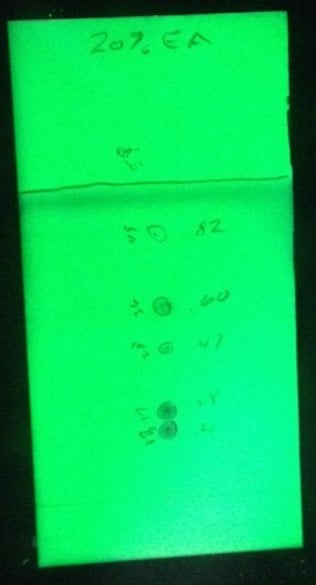
Figure 2. TLC separation in 20% EtOAc shows a classic figure 8 separation of the bottom two spots. Compounds eluting this closely can be difficult to separate by flash chromatography.
The TLC results show that the bottom two compounds elute closely to each other in a classic figure 8 pattern which makes them a bit more difficult to separate and will minimize loading capacity, Table 1.
Table 1. Rf and CV calculations
| Rf | CV | |
| Compound 1 | 0.82 | 1.22 |
| Compound 2 | 0.6 | 1.67 |
| Compound 3 | 0.47 | 2.12 |
| Compound 4 | 0.29 | 3.45 |
| Compound 5 | 0.22 | 4.54 |
Using my flash system’s TLC to gradient capability a method consisting of a 5% to 40% EtOAc linear gradient was created from the above TLC. My load was 100 mg and my columns were Biotage® ZIP 10 gram silica columns
The linear gradient resulted in a separation of all compounds though the last two were not fully resolved, Figure 3.
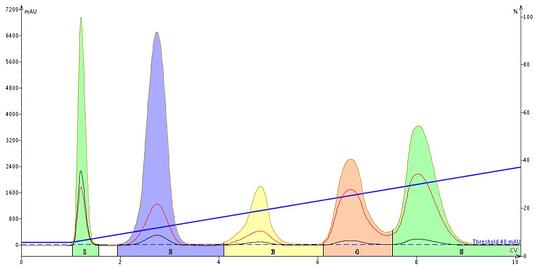
Figure 3. TLC-based linear gradient separated all five compounds though the last two are not fully resolved.
Next, I reran the same gradient but inserted an isocratic hold just as compound four (pink peak) began to elute. The on-the-fly isocratic hold did not improve the separation, Figure 4.
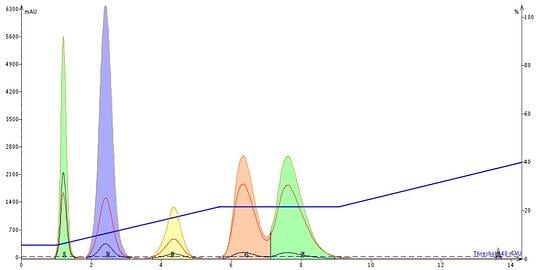
Figure 4. The insertion of an on-the-fly isocratic hold at the beginning of peak 4 did not alter the resolution between it and peak 5 when compared to the original linear gradient.
Based on my logic above, I edited the gradient and built in an isocratic hold 1 CV (column volume) prior to compound 4’s elution; meaning I set the hold to begin at 5 CV. To effectively do this I had to maintain initial gradient slope which, at 3.5%/CV, meant that at 5 CV my % EtOAc in the gradient was 19% (3.5%/CV x 4 CV = 14%; 14% + the initial 1 CV gradient start of 5% = 19%). I set the hold length to 4 CV to ensure it ended after peak 5 fully eluted. The results show this modified method performed about the same as the on-the-fly hold insertion, Figure 5.
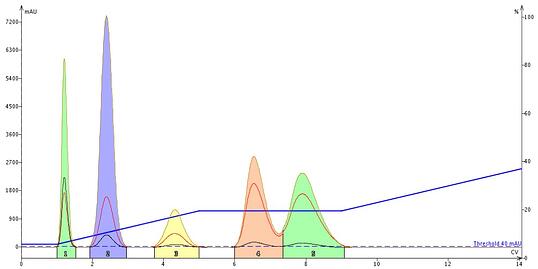
Figure 5. The result of building in an isocratic hold 1 CV before compound 4's elution had minimal impact on its separation from compound 5.
So, giving the situation some further thought I decreased the isocratic hold starting point to 3 CV. Why set it at 3 CV? Well, basically it was a hunch. Based on this new earlier starting point I set the EtOAc target in the gradient to 12% but increased the hold volume to 6 CV to compensate for the additional 2 CV from the now earlier hold implementation point.
Well, low and behold, when I ran this method there indeed was an improvement in the separation of compounds 4 and 5, Figure 6. I believe that the reason may be tied to re-establishing equilibration with the new solvent conditions.
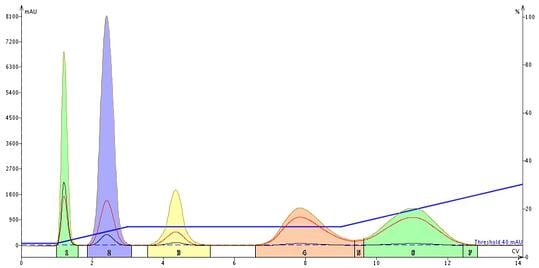
Figure 6. Programming an isocratic hold 4 CV prior to the elution improves the resolution to nearly baseline between peaks 4 and 5.
Dry flash cartridges are typically equilibrated with a minimum of 3 CV and this data suggests this is a minimum volume requirement when programming an isocratic hold into a linear gradient. Based on this theory, my suggestion is to insert the isocratic hold 3 CV prior to the expected beginning of the elution for the lead compound in a low resolution pair of compounds.
So, if you have not run the purification before, and just have TLC data, how can you estimate the point in time during the gradient when the hold should be inserted?
I believe the answer exists within your TLC data. In table 1 above compound 4 has an Rf of 0.29 (center of the spot) which equates to a CV of 3.45. What this means is that if we ran the purification in 20% ethyl acetate compound 4 will elute with its apex approximately 3.45 CV. Compounds always elute in bands which are graphically displayed in Gaussian-type curves so detection starts earlier.
The fact that we are eluting with a gradient delays each compound's elution volume which gives us some freedom to insert an isocratic hold somewhere if needed. My theory is that if you multiply the calculated CV from your TLC data by 2 you will be able to approximate the number of CV required for that compound’s apex to elute in the TLC-based gradient. Once that number is determined, set your isocratic hold 4 CV prior to that compound’s predicted elution CV (apex). Setting it 4 CV ahead will compensate for the peak’s leading edge and ensure the cartridge re-establishes equilibration.
The pertinent equations to help determine the isocratic hold starting point are below
CV = 1/Rf
Hold start (CV) = (CV*2) - 4
In my sample, compound 4 has a CV of 3.45 based on its TLC Rf value of 0.29. Doubling that gives us a value of 6.9 CV (actual elution volume at the peak apex in the full linear gradient was 6.7 CV). Factoring in 1 CV for the beginning of the peak and starting the isocratic hold at 3 CV provided sufficient time for the cartridge to re-equilibrate in the isocratic solvent mix and positively impact the separation.
For my sample as described above...
CV = 1/Rf = 1/0.29 = 3.45
Hold start = (CV*2) - 4 = (3.45*2) - 4 = 2.9 CV
So, this calculation says to insert the isocratic hold in your programmed gradient method at 2.9 (or 3) CV.
Interested in learning more about flash chromatography? Download our whitepaper Inspiring Productivity with Modern Flash Chromatography.

