This is a question I asked myself while I have been studying synthesis variables to see what, if any, impact each variable has on reaction product yield and purity. For this post, I evaluated the order in which I added reactants and solvent.
My colleague, Beth Denton, who is our resident peptide expert, says it does impact her peptide products. But does this hold true with small molecule synthesis as well? To quote a very old TV commercial, “enquiring minds want to know”; so, did I.
My reaction was simple – isatoic anhydride + benzylamine with the solvent being water, yes water, Figure 1. Though only benzylamine has solubility in water, heated water has a lower dielectric constant which helps increase its ability to solubilize organic compounds. For these experiments, two reactions were conducted where the addition order was varied.
- -Isatoic anhydride + benzylamine + solvent
- -Isatoic anhydride + solvent + benzylamine
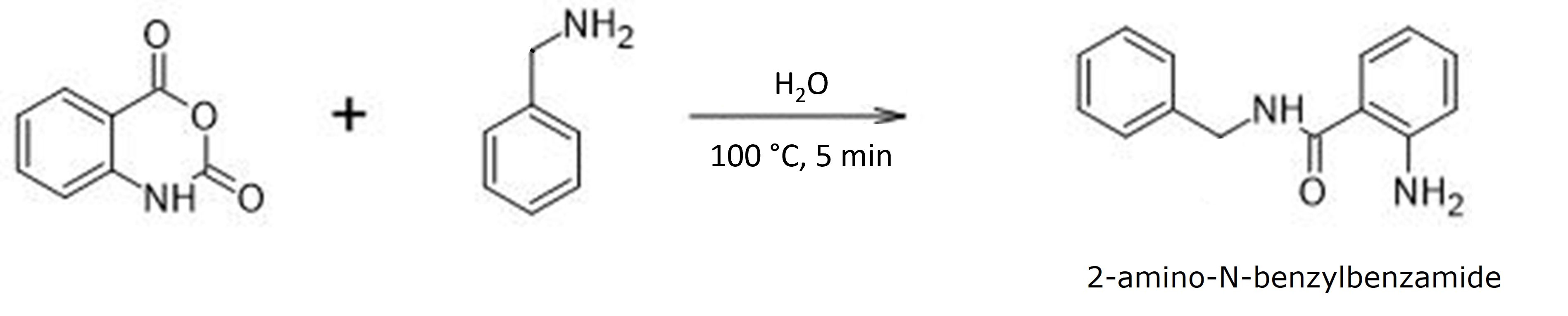
Figure 1. Isatoic anhydride reaction with benzylamine in water at 100 °C for 5 minutes yielded 2-amino-N-benzylbenzamide.
The first reaction set was exothermic and cooled when solvent was added. The second set, where solvent was added to the anhydride prior to the base, did not show signs of noticeable warming.
To facilitate the reaction, I used a Biotage® Initiator+ microwave using ~250 mg of anhydride and ~250 mg of amine with ~4 mL of water. The reaction was performed for 5 minutes at 100 °C and allowed to cool prior to removing the vial’s cap. Since the reaction product was organic, it required extraction with DCM and an ISOLUTE® Phase Separator.
Curious to know crude yield, I evaporated the DCM extracted products (Biotage® V-10 Touch) into tared scintillation vials. The yields were almost the same, Table 1.
Table 1. Crude reaction yields, in grams, post extraction. IA = isatoic anhydride, BA = benzylamine.
My next step was normal-phase flash chromatography. Thin-layer chromatography showed my product having an Rf of 0.21 in 20% ethyl acetate/hexanes with no closely eluting by-products. This should be an easy purification with a high load so I used a 5-gram silica column (Biotage® Sfär HC) and dry loaded the entire crude using silica as the sorbent, about a 7% load by weight.
Wondering if other compounds were co-eluting with my amide reaction product, I used a Biotage® Isolera Dalton 2000 mass-directed flash purification system and targeted four m/z+H masses, +226.6 (product), +314.8, +266.8, and +372.8, all which were detected in the crude mixtures by the Dalton 2000 prior to purification.
So far, so good. Crude yields were very similar and the mass-detectable by-products are the same indicating no differences.
The purification results? Also, very similar but the detected by-product masses ALL co-eluted with my product, which retained some tan color from at least one other by-product, Figure 2.
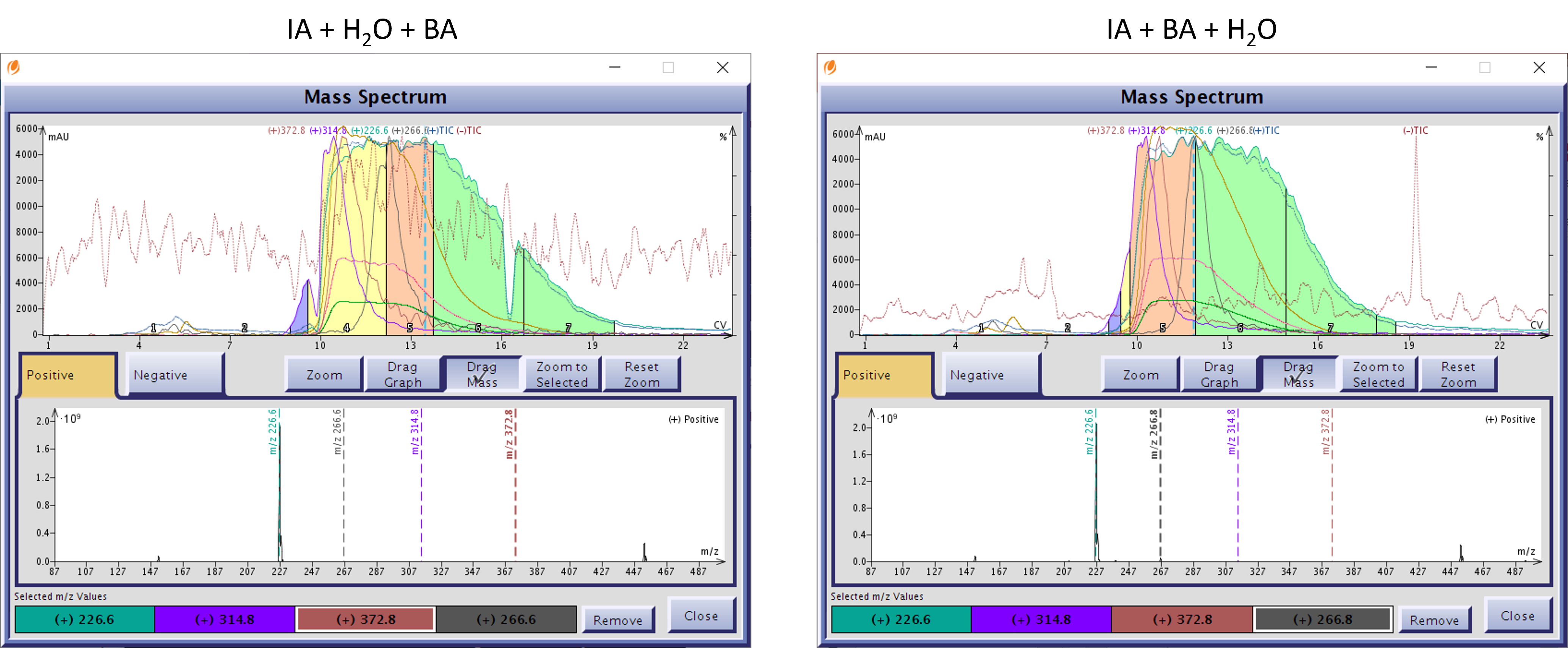
Figure 2. Normal-phase flash purification results of isatoic anhydride + water + benzylamine reaction (left) and isatoic anhydride + benyzylamine + water reaction (right) are nearly identical with the same three co-eluting by-products, +m/z+H 314.8, 372.8, and 266.8.
When I encounter these situations, I typically re-purify the product fractions by reversed-phase because, more often than not, what normal-phase can’t separate, reversed-phase can. This technique of first using normal-phase and re-purifying the isolated product by reversed-phase is known as orthogonal flash chromatography. This technique is very helpful when you need to remove by-products and impurities not resolvable by normal-phase.
In preparation for reversed-phase flash chromatography, the normal-phase fractions containing my amide product were evaporated and weighed, providing very similar yields, Table 2.
Table 2. Normal-phase purified product yields for both reaction orders in grams.
For reversed-phase flash I again used dry loading (on silica) and ran a 35-80% methanol gradient with a 12-gram Sfär C18 column. The results showed that two of the three co-eluting by-products were separated from my amide product with only the by-product with m/z+H 266.8 still co-eluting. While detectable, the amount of the coeluting compound was very low in comparison to the product as can be seen in Figure 3.
Figure 3. Reversed-phase purification of the normal-phase isolated product peak from both reactions shows removal of the by-products with m/z+H 314.8 and 372.8. Left - anhydride + water + amine. Right - anhydride + amine + water.
Not perfect, but a far sight better than what normal-phase provided.
I dried and weighed the product fractions which again provided almost identical yields, Table 3.
Table 3. Reversed-phase flash purification product yields (grams) with % recovery.
So, for this amide reaction conducted in water, the order in which reagents and solvent are added makes no difference. The purified product from both reactions were bright, white crystalline solids with nearly identical yields, Figure 4.
Figure 4. White crystals from both orthogonally-purified amide reaction products.
If you are interested in reading about some of the results of some of the other variables I have addressed with this synthesis, please visit our blog site by clicking the link below.