The issue of greener synthesis workflow is becoming more important as chemists, and the institutions that employ them, evaluate various ways to reduce organic solvent use in both synthetic and chromatographic methods. For synthesis, the most addressed variables include reaction temperature, reaction solvents, time, scale, and catalyst choice as the chemist tries to find the best yield for their desired product using minimal solvent and time. The same is true with the reaction mixture purification – speed, loading capacity, and method simplicity being the most common in need of optimization.
In this post I share the results of some recent experimentation where a variety of solvents were used in a synthesis but reaction scale, time, and temperature were held constant and purification was conducted using highly green reversed-phase flash chromatography.
My reaction was fairly simple – isatoic anhydride reaction with α-methylbenzylamine (in a 1:2 molar ratio) at 150 °C for 15 minutes using microwave heating (Biotage® Initiator+), creating 2-amino-N-(1-phenylethyl)benzamide, Figure 1. This synthesis is similar to one I had posted previously where I also evaluated an array of reaction solvents but that research did not include water.
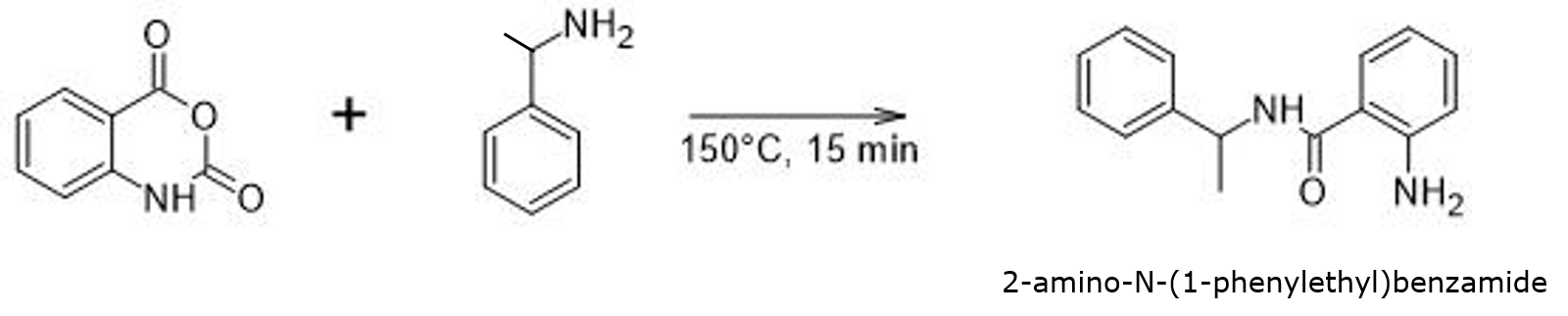
Figure 1. Isatoic anhydride reaction with α-methylbenzylamine performed via microwave for 15 minutes at 150 °C creating 2-amino-N-(1-phenylethyl) benzamide.
For this reaction, I used approximately 280 mg of isatoic anhydride with approximately 400 mg α-methylbenzylamine mixed with ~4 mL of solvent in a sealed 2-5 mL reaction vial.
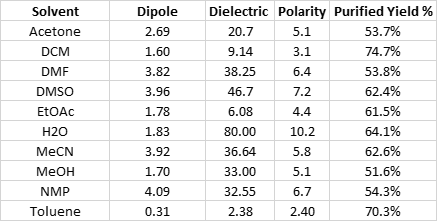
Besides water, the other solvents evaluated included acetonitrile, ethyl acetate, N,N-dimethylformamide, N-methylpyrrolidone, methanol, dimethylsulfoxide, acetone, toluene, and dichloromethane. I chose these reaction solvents because of their dipole, polarity, and dielectric constant differences, Table 1.
Table 1. Reaction solvent properties.
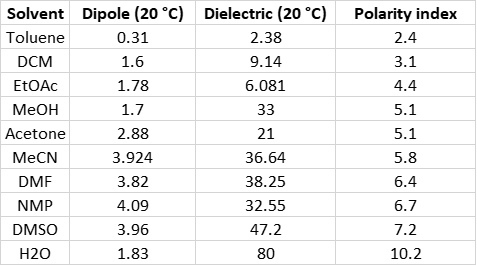
As you can see, dipole moments range from 0.31 (toluene) to 4.09 (NMP) while the dielectric constants vary greatly. Dipoles and dielectric constants in microwave reaction chemistry are important because to be heated by microwave energy, the solvent must have a permanent dipole [1] and absorb microwave (dielectric constant). The heated solvents transfer their heat to the reactants (if they do not themselves absorb the energy) causing them to collide which generates friction and pressure while increasing reaction rates.
The organic solvents’ dielectric constants range from a low of 2.38 (toluene) to a high of 47.2 (DMSO) which basically falls in-line with their polarity [2]. Solvents with higher dielectric constants and polarity tend to heat more rapidly than those with lower values.
Water, as you can see, has a far higher dielectric constant and polarity than any of the other solvents and is typically poor at dissolving most organic compounds. However, when heated, water’s dielectric constant decreases and at 150 °C, has an estimated value of 42.5, giving it pseudo-organic solvation properties [3]. This value (42.5) was determined by plotting the published water dielectric constant vs. temperature data in Lange’s Handbook of Chemistry [4] plus a data point at 300 °C from reference 3, Figure 2.
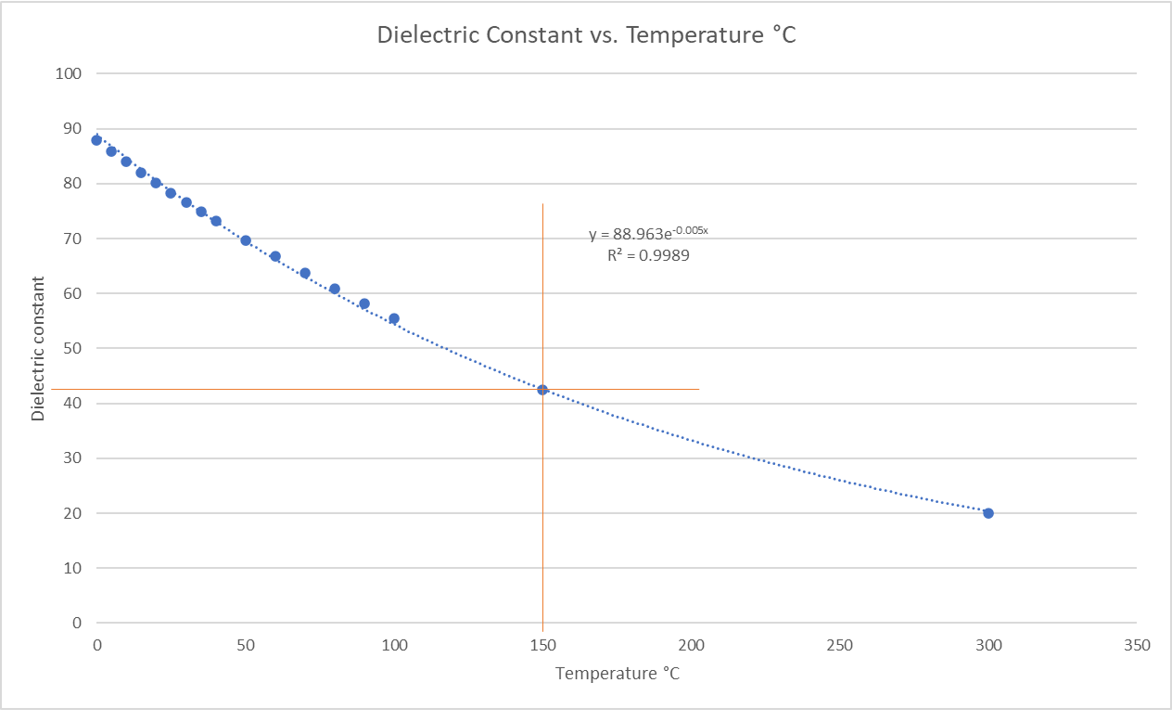 Figure 2. Water dielectric constant vs. temperature graph shows that at 150 °C, water's dielectric constant is ~42.5.
Figure 2. Water dielectric constant vs. temperature graph shows that at 150 °C, water's dielectric constant is ~42.5.
Now that the background has been covered, I will share my data which was quite enlightening to me.
In all reactions, except the one using water, the final reaction solution was a brown oily liquid. With the water reaction, the product, and its by-products, were a brown, insoluble oil that stuck to the reaction vial. Flash chromatography of the water showed product was made but that little was present, Figure 3.
/Blogs%20ONLY/Water%20reaction%20analysis-4.png?width=819&height=408&name=Water%20reaction%20analysis-4.png) Figure. 3 Water reaction solvent analysis shows the product and major by-product present at low levels.
Figure. 3 Water reaction solvent analysis shows the product and major by-product present at low levels.
The brown oil was extracted with a Biotage® Phase Separator and DCM. To accomplish the extraction, I transferred the aqueous portion of the reaction mix to the phase separator. I then added DCM to the reaction vial contents and transferred the dissolved product to the phase separator, which only allows DCM to pass (water cannot permeate the hydrophobic filter). Three DCM washes of the reaction vessel were added to the phase separator with the DCM extract collected into a tared 20 mL scintillation vial.
The contents of the other solvents’ reaction vials were also extracted using the same process, collecting the extract into tared 20 mL scintillation vials each of which were dried using the Biotage® V-10 Touch. Each dried reaction mixture was a thick, brown oil. However, the water reaction’s dried oil showed crystal formation – a different phenomenon from the others. Crude reaction yields, post extraction and prior to purification, ranged from 451 mg (MeOH reaction) to 535 mg (DMF reaction); the water reaction yield was 463 mg, Table 2.
Table 2. Extracted reaction yield in grams.
Each dried reaction mixture was purified by reversed-phase flash chromatography (Biotage® Selekt) with a 12-gram Biotage® Sfär C18 column and external dry load vessel using Biotage® KP-C18-HS as the dry load sorbent. The gradient was 35-85% methanol in water over 10 column volumes (CV). Compounds were fractionated by UV (λ-all 200-400 nm).
Chromatographic results showed each reaction generated the desired product and several by-products, Figure 4.
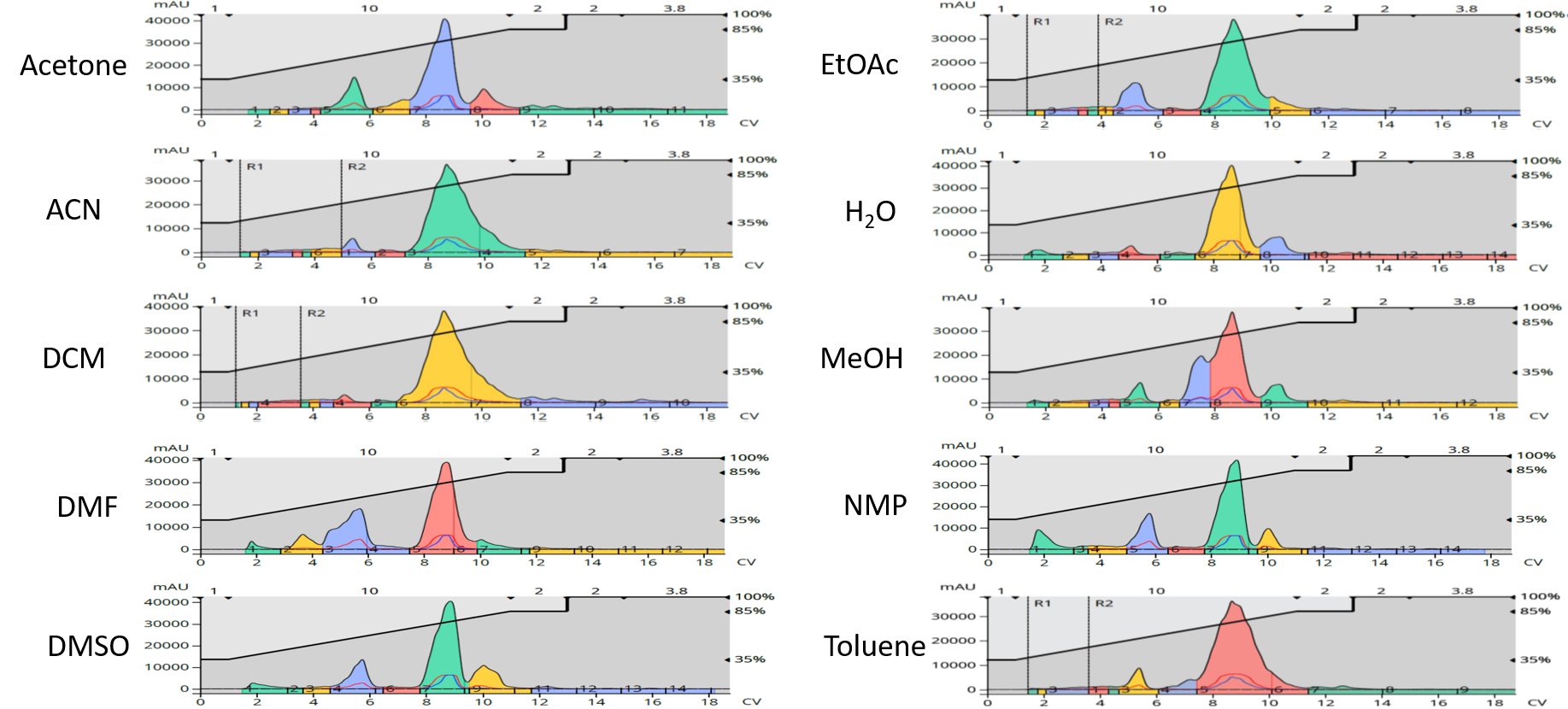
Figure 4. Reaction purification composite show the water-solvated synthesis had among the fewest by-products (along with acetonitrile and dichloromethane).
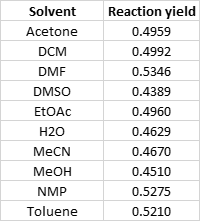
Crude product purity, based on UV, was highest with the ACN reaction (73.0%) with DCM next best with 71.7%. The product purity from the water solvated reaction was 62.2%. The lowest purity product was generated in the methanol synthesis (45.8%), which was the only reaction to create a major by-product eluting at the front of the product peak (alcohols react with anhydrides so this is no surprise). Likewise, purification yields were best in MeCN (77.0%), DCM (74.7%), toluene (70.3%), and water (64.1%). None of purified yields were below 51%, Table 3.
These results show that while dipole, polarity, and dielectric properties are needed to support a chemical reaction, the actual values do not seem to play a role in synthetic yield or purity, especially if the reactants absorb microwave energy, as in this reaction.
Table 3. Purified product yield compared to reaction solvent properties.
So, to answer the main question, yes, water can be effectively used as a reaction solvent especially when heated above its boiling point and under pressure. You will need to extract, but the amount of DCM is low and removes many impurities.
Coupled with reversed-phase flash chromatography, this is a very green and sustainable synthetic workflow.
Click here for more information on reversed-phase flash chromatography (UI468 reversed-phase loading capacity whitepaper)
[1] Microwave Chemistry in Organic Synthesis.pdf (ucla.edu)
[2] Solvent Physical Properties (umass.edu)
[3] A brief review: Microwave assisted organic reaction. Madhvi A. Surati, Smita Jauhari, K. R. Desai, Scholars Research Library Archives of Applied Science Research, 2012, 4 (1):645-661
[4] Lange’s Handbook of Chemistry, 15th ed. John A. Dean, p 5.134

