Though this is a purification blog I do, from time to time, like to address synthetic chemistry experimentation findings in the desire to assist you with your reactions, as this is the front-end of your synthesis workflow. So, in this post, I report on some findings of the effect of reaction temperature on the synthesis of an amide, 2-amino-N-benzylbenzamide, a potential antibacterial compound [1].
The reaction used isatoic anhydride and benzylamine with a microwave system designed for chemical synthesis (Biotage® Initiator+). Reactions were performed at different temperatures with two very different solvents – ethyl acetate and water, Figure 1. That’s right, water.
Previous research I conducted, and posted, for a very similar reaction proved water acts as an excellent heat conductor and allows the bi-phasic reaction to occur with fewer by-products than syntheses performed with most organic solvents.
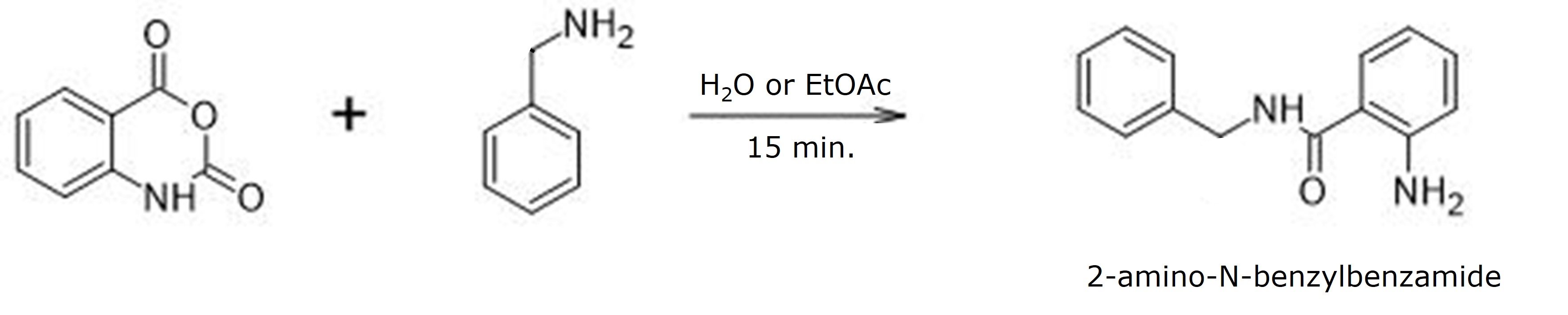
Figure 1. Reaction of isatoic anhydride with benzylamine produced 2-amino-N-benzylbenzamide. Solvents used were either water or EtOAc. Temperatures varied from 75 °C to 200 °C.
For this synthesis, I varied reaction temperatures from 75 °C to 200 °C in 25 °C intervals while keeping the synthesis duration to 15 minutes. Post synthesis, all reactions were extracted with 5 x 2 mL DCM to ensure both reactions followed the same basic protocol to maintain an “apples-to-apples” comparison; the reaction product and by-products are not water soluble but are EtOAc soluble.
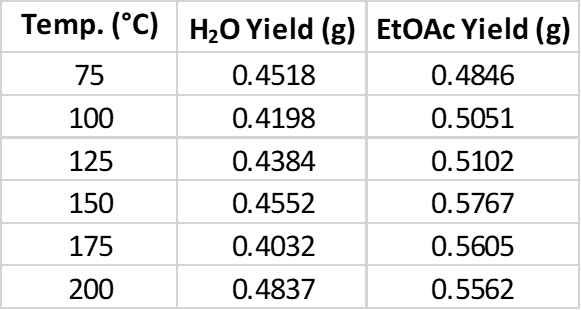
For extraction I used ISOLUTE® Phase Separator cartridges, which take the place of your typical separatory funnel. The DCM extracts were evaporated using a Biotage® V-10 Touch rapid solvent evaporator system. Reaction yields were lower with the water-based syntheses, possibly due to ethyl acetate’s ability to dissolve more by-products, Table 1.
Table 1. Isatoic anhydride + benzylamine reaction yields for water and EtOAc.
Flash chromatography (reversed-phase) was used to assess product purity by loading 8-10 mg on a 12-gram Biotage® Sfär C18 column, Figure 2.
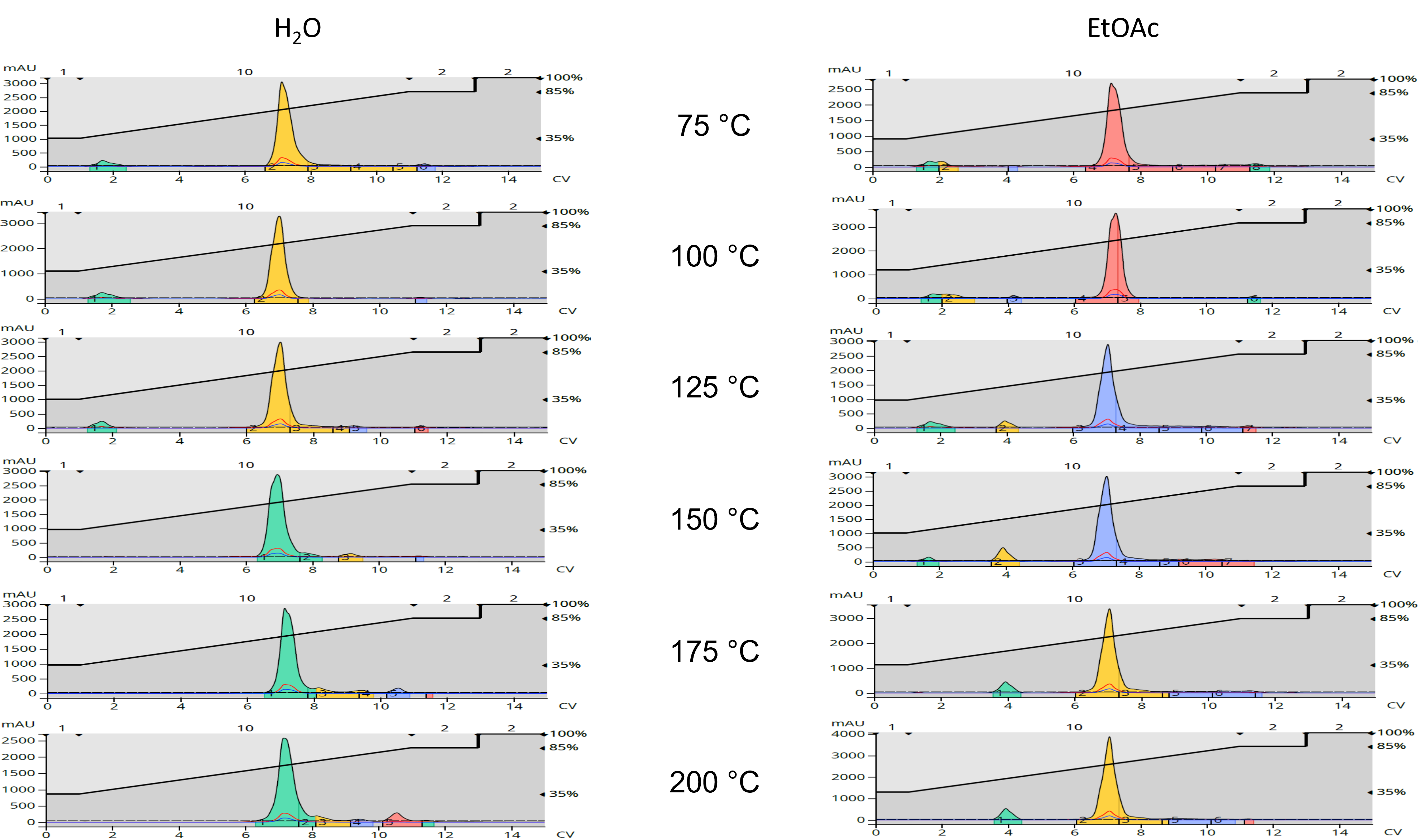
Fig. 2 Isatoic anhydride + benzylamine flash chromatography comparisons - water (left) and EtOAc (right).
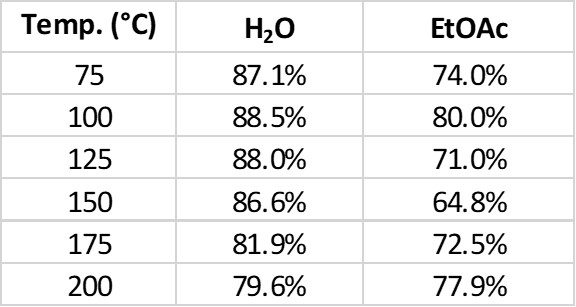
The flash chromatography data, using a Biotage® Selekt system, showed the water-based reactions being purer than the ethyl acetate-solvated reactions at every temperature, Table 2.
Table 2. Crude reaction product purity at different temperatures.
Both the EtOAc and water reactions at 100 °C provided the highest purity product and were purified using reversed-phase flash chromatography (12-gram Biotage® Sfär C18, 35-85% methanol over 10 CV, dry load with Biotage® KP-C18-HS). The chromatograms showed complete separation of the product from its nearest eluting by-products, even at relatively high loads (water reaction = 3.5% load by weight, EtOAc reaction = 4.2% load by weight), Figure 3.
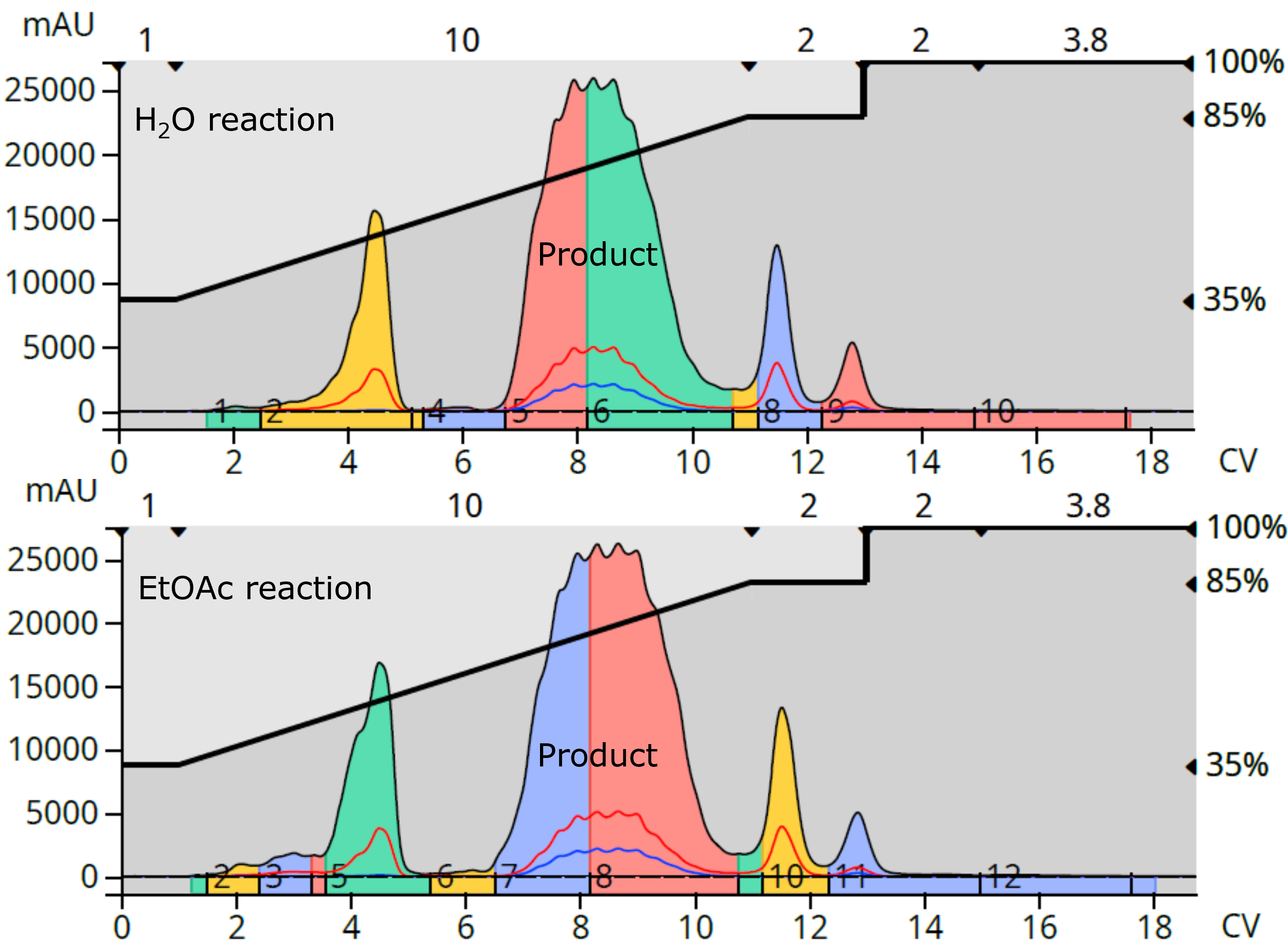
Fig. 3. Flash purification comparison of the water reaction (top) and the EtOAc reaction (bottom).
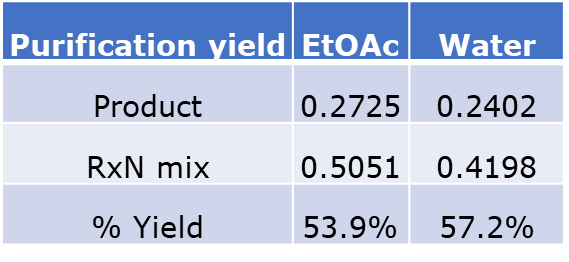
Purified yields were 0.2402 grams for the water reaction and 0.2725 grams for the EtOAc reaction, which equates to 57.2% and 53.9%, resp., Table 3.
Table 3. Comparison of purification yields.
So, what does this data tell us? It tells me that when performing this reaction, and likely other amide-creating reactions, that…
- 1. Solvent choice does matter regarding reaction product purity and yield
- 2. Reaction temperatures do not need to be all that high for amide syntheses
- 3. Water is an excellent “solvent” for microwave-based reactions even when one or more reactants are insoluble
-
For more information on microwave reaction examples, please download our Microwave Reaction Tutorial.
[1] Isonah, U.O. et.al. Synthesis, Characterization, and Antibacterial Investigation of Some Isatoic Anhydride Derived Amides. European Journal of Pharmaceutical and Medical Research, 2021, 8(5), 124-127.

