Jan 23, 2023 3:46:42 PM
Why is TLC Rf important for flash column chromatography optimization?
By Bob Bickler

Many chemists I talk to understand that TLC data is useful for flash chromatography method development. Most also know that they should try to get their target compound to elute with an Rf between 0.15 and 0.4 by adjusting TLC solvent strength. Have you ever wondered why this is important and how Rf values impact flash chromatography results?
In this post I will explain the relationship between TLC Rf and flash elution volumes (CV) and why having your target compound elute in the Rf range is needed.
Since the beginnings of flash chromatography in the 1970's, TLC has been used as the primary flash chromatography method development tool for organic and medicinal chemists. From this early work in flash chromatography a relationship between TLC compound retardation factor (Rf) and flash column elution volume (CV) was determined, Equation 1.
CV = 1/Rf Equation 1
This pioneering research also found that separation quality (selectivity) is better when looking at a difference in adjacent compound CV (ΔCV) rather than adjacent compound Rf (ΔRf).
CV2 - CV1 = ΔCV Equation 2
I have found that this relationship is best explained graphically. In Figure 1, we see that a plot of CV vs. Rf is non-linear. The impact on this non-linear relationship is that for compounds with larger Rf values, the number of CV required to elute them is low. If an adjacent pair of compounds both elute with large Rf this impacts the separation volume (ΔCV) and, therefore the separation quality, fraction purity, and load amount.
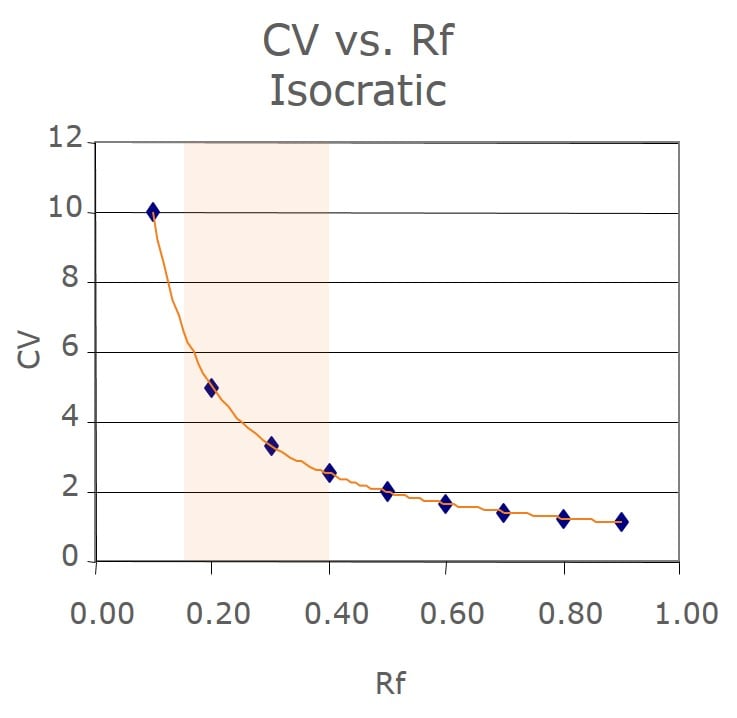 Figure 1. The non-linear CV - Rf relationship impacts separation quality.
Figure 1. The non-linear CV - Rf relationship impacts separation quality.
Adjusting the solvent strength to obtain a low Rf for your target compound and nearest eluting impurities maximizes the separation between them, Figure 2.
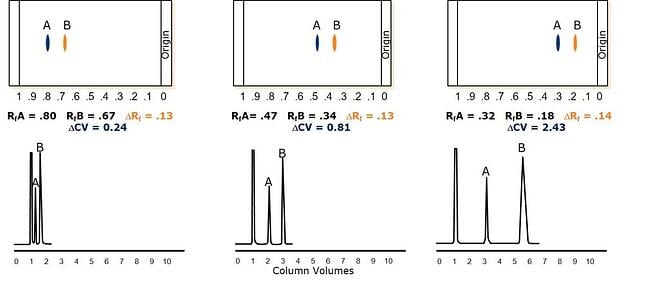 Figure 2. Reducing solvent strength forces sample components to elute later, using a bit more solvent but improving separation quality. Left - high Rf values minimize elution solvent volumes (CV) and separation quality. Middle - solvent strength adjustment to get one compound in the optimal Rf zone provides major separation improvement. Right - Both compounds in optimal zone provides best separation quality.
Figure 2. Reducing solvent strength forces sample components to elute later, using a bit more solvent but improving separation quality. Left - high Rf values minimize elution solvent volumes (CV) and separation quality. Middle - solvent strength adjustment to get one compound in the optimal Rf zone provides major separation improvement. Right - Both compounds in optimal zone provides best separation quality.
In Figure 2 the ΔRf for the two compounds remains constant for each example but as the Rf values for each compound decreases, the amount of separation between them increases. This is represented by increasingly larger ΔCV values which correlate to improved separations and better purity fractions even at higher loads! The reason ΔCV is better gauge of separation quality is because it is volumetric whereas the calculation of Rf, and therefore ΔRf, is strictly a linear distance relationship.
Hopefully, these examples have helped to explain why low Rf values provide better separations.
For more information regarding flash chromatography optimization techniques, download our whitepaper - Successful Flash Chromatography.
Published: Jan 23, 2023 3:46:42 PM

