How the acid dissociation constant (pKa) affects sample preparation method development? Knowing and understanding the pKa of your compounds tells you if the compound can be ionized, and under what conditions, so you can use this property to develop better sample prep methods.
The acid dissociation constant, pKa, is the pH at which an analyte is 50% ionized and 50% non-ionized. Figure 1 show the ionization of an acid and a base with a pKa of 8 across the pH range. For an acid, an analyte is ionized as the pH increases above its pKa, and is non-ionized as the pH is lowered below its pKa. Complete ionization or non-ionization occurs 2 units above or below the pKa. For a base, the compound in non-ionized above the pKa, and ionized below the pKa.
When I need to develop a new sample extraction method, I look up the logP and pKa of the analytes in the assay and consider possible interfering compounds. Two good places to start are chemicalize.com and the human metabolome database.
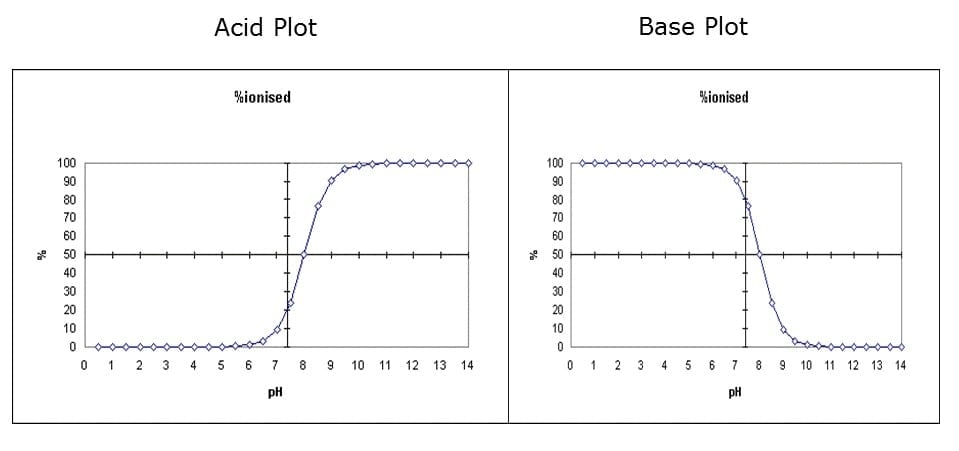 Figure 1. The effect of pH on the ionization of an acid and a base with a pKa of 8.
Figure 1. The effect of pH on the ionization of an acid and a base with a pKa of 8.
If the compounds of interest can be ionized, you can employ strong cation or anion exchange SPE to retain your compounds. These products are usually bonded silica or polymeric phases with strong cation exchange (typically sulfonate) or anion exchange (typically quaternary ammonium) groups, like an ion exchange HPLC column. Since the backbone for either sample prep column is a reverse phase bonded silica or polymer, they also have reverse phase characteristics, so retention by ion exchange or reverse phase can take place using a single SPE column. That’s where the term “mixed mode SPE” comes from. Ion exchange is a very strong retention mechanism. Acidic compounds with a negative charge above their pKa can withstand a high pH aqueous wash, and a strong organic wash prior to elution with an acidified (typically with formic or acetic acid) elution solvent. Basic compounds with a positive charge below their pKa can tolerate an acidic wash, a strong organic wash, and are eluted with an organic solution where a base (typically ammonium hydroxide) is added.
For large urine drug panels with acidic, basic and neutral compounds a single assay, mixed mode polymeric reverse phase strong ion exchange SPE could be your best bet. If you can treat the samples with acid (for bases) or base (for acidic compounds) and ionize them, you can retain the ionized analytes by ion exchange and the non-ionized compounds by reverse phase. Most drugs are basic, so ionizing the basic drugs and retaining the neutrals and acids by reverse phase is a common strategy. You have to be careful about the organic wash with these methods, as the non-ionized compounds retained by reverse phase can be eluted in a strong organic wash. You need to know the logP and the pKa of your compounds, so you know which compounds are retained by reverse phase and which are retained by ion exchange, and how strongly the non-ionized compounds are retained by the reverse phase mechanism. Elution is accomplished with an acidic or basic organic solvent, depending upon the ion exchange mechanism (acidic for anion exchange, basic for cation exchange). The organic solvent also elutes the compounds retained by reverse phase. Choosing the correct organic solvent for washes and elution are important to optimize sample cleanliness. Compromises in sample cleanliness and recovery for some analytes are inherent in methods like these with large numbers of compounds with different properties and retention characteristics.
The take home message is still – KNOW YOUR COMPOUNDS! Look up their logP and pKa values, understand their ionization states and reverse phase retention properties, and formulate your sample prep conditions from this information.
Do you want to know more about sample preparation? We have prepared for you plenty of interesting resources on extraction techniques and method optimization.
For more information on choosing the right sample prep based on compound properties, watch our webinar "Demystifying Sample Prep - A Systematic Approach to Sample Prep Method Development for Clinical, Forensic and CRO Laboratories".

