For chemists preferring or needing to dry load their crude sample mixtures to get an acceptable flash purification result, using the right ratio of sample to sorbent can be quite important. Too much sample and solubility issues can ensue, too little sample and significant band broadening occurs, reducing the separation quality.
In this post, I propose an acceptable ratio range based on my own experimental data.
What is dry loading? For those who may be unfamiliar with the term, dry loading is a common flash chromatography loading technique used to facilitate purification of poorly soluble compounds and samples dissolved in chromatographically strong solvents.
For most chemists, liquid loading is used because it is quick (I often use it). However, liquid loading can also be problematic. If the dissolution solvent is too strong, it can interrupt the sample components' affinity for the column's media and distort what should be a good purification.
Dry loading eliminates these issues, at least in theory. However, even with dry loading there are some caveats mostly centered around the sample/sorbent ratio.
Let's explore the following caveats:
Ensure the sorbent is inert towards the target compound- Maintain a suitable ratio of sample to sorbent
Silica is most commonly used as a sorbent but may not be the best choice due to chemical interactions and irreversible adsorption. Other media such as diatomaceous earth Celite® (or Biotage® HM-N), Florisil®, and alumina are also useful. Even reversed-phase media and ion exchangers can be used.
When using silica, Florisil, or alumina (all relatively high surface area media), I use a ratio no more concentrated than 1 part sample to 3 parts media (1:3) to 1 part sample to 4 parts media (1:4), by weight not including the solvent.
The reason I recommend this range is two-fold. 1) In my experience, making the mixture more concentrated than 1:3 may lead to broad elution bands and poor resolution. 2) Alternatively, if I choose to make the sample less concentrated than this ratio, I have observed that the sample is spread over too much sorbent and band-broadening (peaks spread) occurs, often deteriorating my separation. In addition, when using diatomaceous earth, which has lower density and surface area, I have found that a greater amount of media can and should be used. For ion exchange media, using a slight excess based on equivalents is best to ensure full ionic binding.
As an example to support my proposal, the data in Figure 1 shows the impact that different sample/silica ratios have on separation quality. A 1:2 ratio actually reduces the amount of separation and a 1:20 ratio, while providing a better separation, also broadens the peaks. At a 1:4 ratio, separation quality is obtained and peaks are more narrow. For this work I used an isocratic hexane/ethyl acetate solvent system.
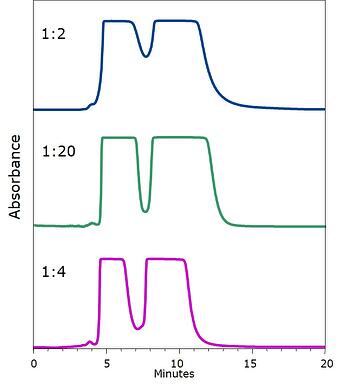 Figure 1. Chromatograms depicting the results of different sample/silica dry load ratios. Top - 1 part sample to 2 parts silica provides insufficient separation. Middle - 1 part sample to 20 parts silica provides a good separation but with broad elution bands. Bottom - 1 part sample to 4 parts silica provides an excellent separation with narrow elution bands.
Figure 1. Chromatograms depicting the results of different sample/silica dry load ratios. Top - 1 part sample to 2 parts silica provides insufficient separation. Middle - 1 part sample to 20 parts silica provides a good separation but with broad elution bands. Bottom - 1 part sample to 4 parts silica provides an excellent separation with narrow elution bands.
These are my results. I am interested in your experiences as well. I will address diatomaceous earth and ion exchangers as dry load sorbents in other posts.
To learn more about flash chromatography, read my white paper on inspiring productivity with modern flash chromatography:

