Our clientele at Biotage have interesting and diverse backgrounds from highly skilled synthetic chemists, to experts in natural product chemistry, to those who are just beginning they journey with chemistry. What I have learned over my 40+ year career in the “art” of chromatography is that for many of these fine folks there is a lack of understanding about chromatographic principles.This lack of understanding, I believe, is only due to their core study curriculum where separation science is used as a tool during lab work but the principles behind why a mixture’s components separate (or do not separate) perhaps are not effectively explained, understood, or studied.
There are four basic types of chromatography including:
- -Gas-liquid chromatography (GC)
- -Liquid-liquid chromatography (LLC)
- -Liquid-solid chromatography (LSC)
- -Size exclusion/gel permeation chromatography (SEC/GPC)
-For this post I will focus on liquid-solid chromatography.
Liquid-solid Chromatography
This is the most popular of the techniques mentioned above because it is useful for semi-volatile and non-volatile compound separations. Since heat is not required to elute compounds from a separating column (as with GC), liquid-solid chromatography requires the use of a solvent or solvent blend of miscible solvents with different polarity to get the sample’s chemical components to separate from each other.
With LSC, the sample is loaded onto a solid support or stationary phase. The sample’s components bind to the stationary phase, but to various degrees. The amount of interaction with the stationary phase controls the separation rate and effectiveness. So, the more attraction to the stationary phase the longer the compounds require to elute. To help elute those strongly bound compounds, the solvent ratio needs to change (increasing strength) over time.
Within the realm of LSC are several different chromatographic techniques…
- -Paper chromatography (PC)
- -Thin-layer chromatography (TLC)
- -High-performance liquid chromatography (HPLC)
- -Flash chromatography (FC)
- -Ion exchange chromatography (IX)
- -Affinity chromatography (AC)
- -Chiral chromatography (CC)
- -Supercritical fluid chromatography (SFC)
In this post I will focus on flash chromatography, the principles of which are the same as HPLC. Both use columns filled with a stationary phase into which sample is injected and through which solvent is pumped. However, flash chromatography uses larger columns than HPLC, which is mostly an analytical separations technique.
Flash chromatography is a preparative chromatographic technique whose columns also are filled with a solid support or media that has a larger particle size than HPLC columns. These media and column size differences enable flash columns to purify more compound per gram of media than HPLC.
- Flash chromatography uses 15 µm to as high as 60 µm media particles
- HPLC uses 1.7 µm to 10 µm media particles
What Happens Inside the Column
Most flash chromatography and HPLC is performed using either normal-phase or reversed-phase methodology. The differences between these methodologies lie in the stationary phase chemistry and solvents utilized to separate a mixture’s individual chemical components.
- Normal-phase uses non-polar and moderately solvents (e.g. hexane and ethyl acetate) for the mobile phase and a polar media (e.g. silica) as the stationary phase
- Reversed-phase is just the opposite, using polar solvents and a non-polar stationary phase
With these methodologies being “polar” opposites of each other (pun intended), the elution order for most compounds is reversed. This is where the chemistry behind chromatographic separations happens.
Let’s focus on normal-phase for now.
Normal-phase chromatography
With Normal-phase chromatography, the chemical compounds comprising a mixture (e.g. reaction mix, natural product extract, etc.) are dissolved in a suitable solvent and injected into the column. When the mixture contacts the polar stationary phase, let’s say silica, the mixture’s compounds and their dissolution solvent compete with the mobile phase for the silica’s binding sites. The more polar the chemical the more strongly attracted to the silica. This attraction is called adsorption (different than absorption).
Absorption is what occurs when a sponge or paper towel interacts with a liquid. Adsorption is a combination of physical and chemical interactions where the silica’s polar surface chemically binds the chemical components it contacts, mostly due to phenomena known as hydrogen bonding and van der Walls forces.
When any chemical interacts with dry silica (as used with a new flash column), adsorption occurs. This interaction liberates heat, which can cause problems with compound stability and chromatographic results. For this reason, silica columns are typically equilibrated (pre-wetted) with a low polarity solvent prior to sample introduction. Low polarity solvents (e.g. hexane, heptane, cyclohexane) are weak solvents and not well adsorbed by silica or other polar media.
Silica’s surface chemistry is a mixture of hydroxyl groups (silanols) and silyl ethers, Figure 1. These functional groups are polar, thought to be negatively charged, and adsorb electron deficient compounds. Aromatic compounds’ pi-clouds can hydrogen bond with the silica’s hydroxyl groups during adsorption.1
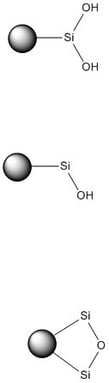
Figure 1. Chromatographic silica's chemistry comprises a mixture of silanols and silyl ether functionalities. These moieties bind with chemicals through hydrogen bonding/adsorption.
To ensure good sample component binding (adsorption), the sample to be purified needs to either be dissolved and loaded into the column in a weak solvent (liquid loading) or, dissolved with a stronger (polar) solvent, mixed with an inert media (e.g. silica, diatomaceous earth), dried, and packed into a different column placed in-line with the purification column (dry loading). Dry loading eliminates many issues encountered when using a liquid load, especially with a sample dissolved in a polar solvent.
After sample load, the mobile phase solvents are introduced. However, to achieve a separation the sample components need to desorb from the stationary phase at different rates. Though this is achievable using a constant polarity solvent (isocratic elution), better purification is typically realized using a mobile phase that starts with low polarity and increases its polarity over time. This is known as gradient elution.
With a gradient, those compounds with higher solubility in the low polarity solvent (lower polarity compounds) desorb first and pass through the column. As the mobile phase solvent polarity increases, adsorbed compounds desorb off the media at different rates based on their solubility in the increasingly polar mobile phase, Figure 2.
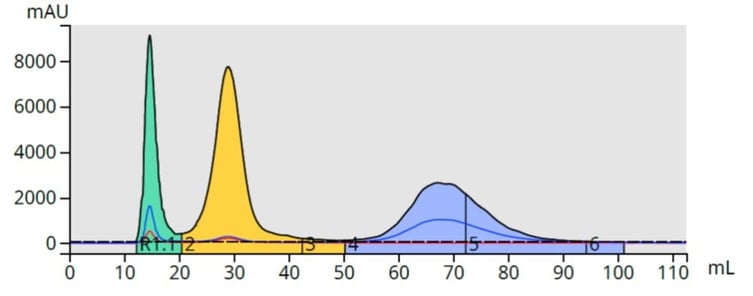
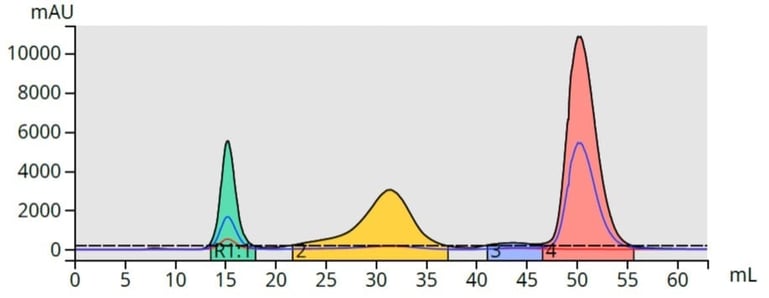
Figure 2. Top - With isocratic elution (constant mobile phase polarity), compounds desorb more slowly than when using a gradient (bottom). Gradient elution typically provides a better separation/purification, than isocratic elution.
An interesting characteristic of normal-phase chromatography is that once a compound desorbs from the media, it never adsorbs again because the increasingly polar mobile phase preferentially adsorbs in its place.
Polarity (binding strength) differences between compounds can be as subtle as the addition of a different functional group, functional group location differences (Parida, 2006) as on an aromatic ring, or as distinct as compounds with totally different structures, Figure 3.
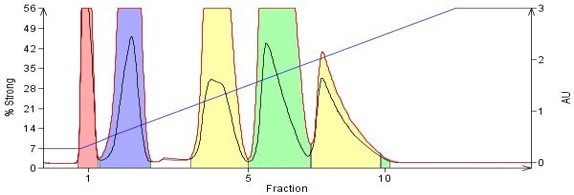
Figure 3. Separation of compounds with differing polarities based on functional group type and location on the molecule. By order of elution - naphthalene (no polar functionality), 1-nitronaphthalene, o-nitroaniline, m-nitroaniline, p-nitroaniline. The nitroanilines differ only in the location of the nitro functional group relative to the amine.
So, to summarize, normal-phase chromatography separations are based on differences in compound polarity (both obvious and subtle), their attraction to the stationary phase, and their solubility in the mobile phase. To obtain the best purification results solvent scouting is recommended using TLC.
Interested in learning more about flash chromatography, download our whitepaper - Successful Flash Chromatography.

