Jan 17, 2023 4:08:03 PM
The Hidden Dangers of Organic Solvents
By Maura Rury

“Our laboratory uses organic solvents every day. Should we be concerned about solvent exposure?” I hear this question fairly often and the short and simple answer is: YES. But if this were a simple yes/no question, I wouldn’t have anything else to say, and this would be the shortest blog post that’s ever been written.
The truth is, every laboratory owner, director and manager should be concerned about the risk that solvent exposure poses to their chemists and technicians; but it’s not the solvent itself that we’re most concerned with. Yes, an accident could occur that would result in a chemist being covered in one or more chemicals. But those types of accidents are (hopefully!) relatively rare. The typical, more common exposure I’m referring to is not to the solvent, but to the solvent vapors.
Organic solvents are commonly used in laboratories, regardless of the specific chemistry or lab work that’s being done. Solvents are grouped into this class of compounds if they contain at least 1 carbon atom and at least 1 hydrogen atom. They also have similar physical properties such as:
- State and color – clear, colorless, and liquid at room temperature
- Boiling point – low boiling point and easy to evaporate
- Volatility – highly volatile and easy to vaporize, which is why they’re known for producing a strong (and somewhat offensive) smell at room temperature
Their low boiling point and high volatility are the driving reasons these compounds are referred to as volatile organic compounds (VOCs). The U.S. EPA and World Health Organization (WHO) take this definition a step further and subdivide VOC compounds into 3 categories, based on boiling point ranges. These compounds are defined as “very volatile organic compounds” (or “wicked volatile organic compounds,” if you’re from the New England area), “volatile organic compounds” and “semi-volatile organic compounds.” A breakdown of this classification is listed in the table below:
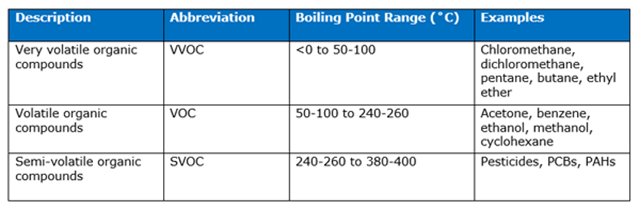
VVOC Compounds
As the name implies, VVOC compounds have the lowest boiling points and are very volatile. These compounds are so volatile, they can be difficult to work with in the lab if you need them to be in the liquid state. These compounds readily vaporize and, if you’re not careful, an entire flask, beaker or bottle of one of these solvents could convert to the gas phase. Not only will your lab work grind to a halt because you’ve lost your solvent, but your safety is at risk. Solvent vapors are hard to capture and confine so there’s not much to stop them from surrounding you or finding a nearby heat source in the lab and igniting into a giant fireball.
VOC Compounds
VOC compounds are in the mid-range for volatility. They’re less volatile than VVOCs, but more volatile than SVOCs. The boiling point range for this class of compounds is quite large, but these compounds will still evaporate quickly if you leave it sitting out at room temperature. These compounds need to be handled carefully, but they’re usually easier to work with (compared to VVOCs) in terms of keeping them from evaporating and escaping into your lab. In fact, if you’ve ever used a can of paint to repaint a wall or opened a bottle of acetone to take the nail polish off your fingernails, you’re familiar with some of the safe handling practices of VOC compounds. Generally speaking, these compounds will stay in their original container, as long as you’re using an appropriate container and you keep it tightly sealed.
SVOC Compounds
SVOCs are the class of VOC compounds with the highest boiling points and lowest volatility. The name can be misleading because the boiling point range for SVOC compounds is quite high and this category includes compounds that are used in flame retardants. Yes, you read that correctly. Compounds that are classified as “semi-volatile” are sometimes used for slowing or preventing fire. If you’re working with SVOCs in the lab, you’re generally not using them as solvents. You’re generally using other (more volatile) organic solvents to extract these compounds from a solution. Even though SVOCs are less volatile than their VVOC and VOC counterparts, their hazard is no less significant. The hazard from these compounds is just more likely to come from physical contact, rather than from inhaling vapors.
Regardless of the volatility of the solvents you’re working with, you should always wear the proper protective equipment and work with them in ventilated, climate-controlled, environments. Volatile organic solvents pose an extra risk as the vapors can become hazardous even before you’ve realized you have a problem. If you’re using volatile organic solvents to extract SVOC compounds, make sure your extractor is set up to pull solvent fumes away from you and vents those vapors into a fume hood. This is easiest to accomplish by operating the extractor directly in the fume hood; however, if this isn’t an option, make sure a proper vent hose is connected to transport the solvent vapors from the system into the closest fume hood. You can take additional steps to reduce exposure to solvent vapors with vapor shields that are designed to prevent solvents from splashing and from solvent vapors from escaping. Download our technical note to see an example of one and make sure you’re maximizing your laboratory safety!
Published: Jan 17, 2023 4:08:03 PM

