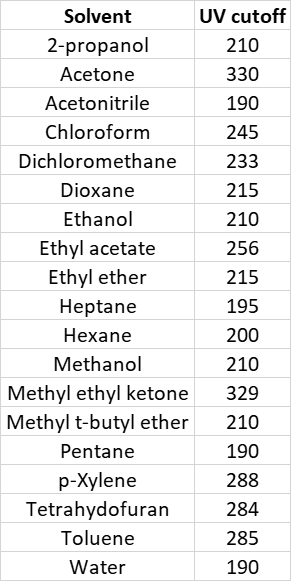
Flash chromatography with real-time UV detection and fractionation is the primary tool of chemists for isolating synthetic compounds. However, many commonly used flash chromatography solvents (ethyl acetate, dichloromethane, toluene, and acetone) absorb UV light, often in the detection range of either the target compound, byproducts, or both, Table 1. This issue, as you can imagine, can cause problems with product recovery and even purity.
Table 1. Common chromatography solvents and the UV-cutoff
So, how do you detect compounds that absorb at the same wavelengths as the solvents being used? Well, one useful option is to use your flash system’s baseline correction feature, if equipped. Baseline correction will remove the chromatography solvent’s UV absorption and provide a flat baseline enabling detection of more compounds in the mixture.
But what do you do if baseline correction is not an option or your target compounds’ molar absorbance is lower than that of the UV absorbing solvent? Well, you can replace the UV absorbing solvent with a transparent solvent or switch to reversed-phase flash chromatography that uses UV-transparent solvents.
Let’s take a look at the value of switching solvents with the purification of a synthetic reaction mixture, Figure 1.
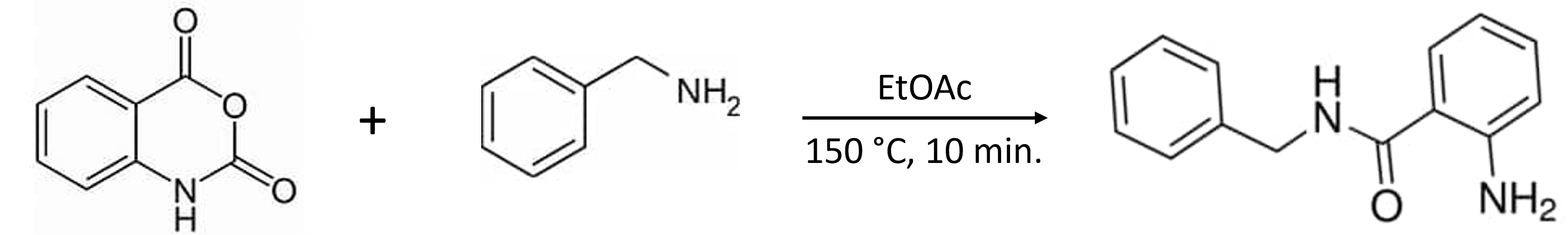 Figure 1. Reaction of isatoic anhydride and benzylamine
Figure 1. Reaction of isatoic anhydride and benzylamine
Reaction mixture purification is commonly performed using a hexane or heptane with ethyl acetate (EtOAc) gradient. This reaction’s product absorbs UV between 200 and 400 nm with maxima around 254 and 330 nm. The reaction’s byproducts, however, likely have lower concentrations and other detection wavelength maxima, which could be obscured by the UV absorbing solvent e.g., EtOAc. If these byproducts are not detectable, decreased product purity and yield are possible.
For this reaction mixture a 10 mg aliquot of the reaction mixture was purified using a 5-gram Biotage® Sfär HC column. A Biotage® Selekt flash chromatography system was used with a linear 7-60% EtOAc in heptane gradient at a flow rate of 18 mL/min and a detection wavelength range of 198-810 nm (without baseline correction), Figure 2. As shown in Table 1, EtOAc absorbs UV light from below 200 nm to 255 nm [1] so compounds absorbing UV in this range may not be easily detected.
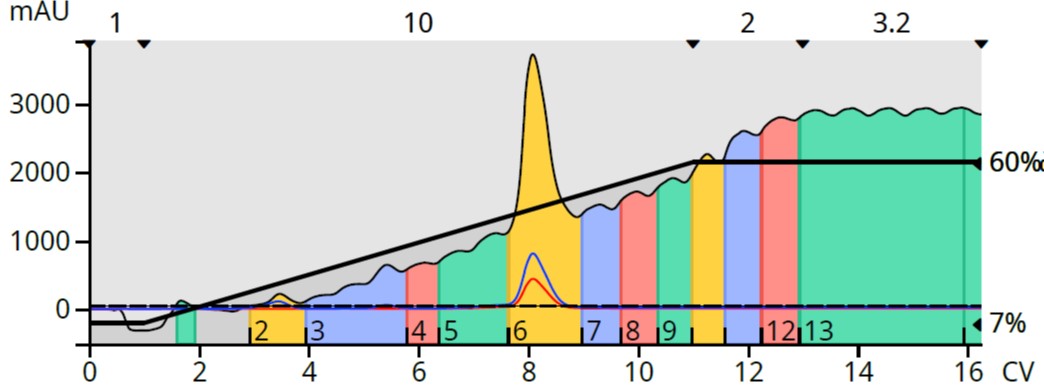 Figure 2. Reaction mixture flash chromatography using a heptane/ethyl acetate gradient with UV detection without baseline correction.
Figure 2. Reaction mixture flash chromatography using a heptane/ethyl acetate gradient with UV detection without baseline correction.
This chromatogram clearly shows a rising baseline as the gradient progresses. While the product peak is visible, none of the by products are detectable. Though it is possible that there are no byproducts, that scenario is highly improbable.
To determine if and where the byproducts eluted, ethyl acetate was switched to methyl t-butyl ether (MTBE), which is UV transparent across all wavelengths. MTBE also has the same solvent strength as EtOAc (0.48) [2] so the gradient did not require alteration, Figure 3.
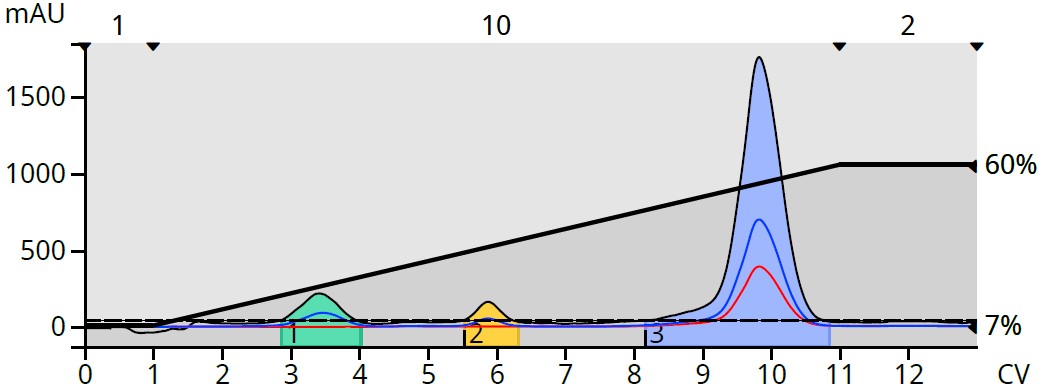 Figure 3. Reaction mixture purification using a heptane/MTBE gradient revealed hidden byproducts.
Figure 3. Reaction mixture purification using a heptane/MTBE gradient revealed hidden byproducts.
The switch from UV absorbing EtOAc to UV transparent MTBE eliminated the rising baseline and improved the detectability of not only the target product but also the generated byproducts.
If detecting and removing byproducts from your reaction mixture is important to you, consider using UV transparent solvents in place of those that are UV absorbing.
For more flash chromatography tips, download our whitepaper - Successful Flash Chromatography.
[1] Dean, John A. Lange’s Handbook of Chemistry, 15th edition, pp.11.16-11.17
[2] Snyder, L., Kirkland, J.; Introduction to Modern Liquid Chromatography, Appendix 1, Properties of HPLC Solvents, Wiley, 2009.

