Many chemists today find they need to synthesize molecules at higher temperatures in order to force difficult reactions to proceed. Solvents such as DMF, DMSO, and NMP are commonly used in these reactions as they facilitate the use of the high reaction temperatures. However, the same attributes that make these chemicals attractive as reaction solvents make compound recovery from them very difficult, including flash column chromatography. These high boiling solvents are typically polar and pose a challenge if purification is to be accomplished with normal-phase silica.
In this post I explore some options that enable purification of high boiling point solvated reaction mixtures through simple sample work-up.
Since reaction volumes tend to be large compared to the amount of product synthesized, direct solvent injections are not always practical or warranted, especially with polar, high boiling solvents. The polar character and sheer volume of these solvents impact the adsorption/desorption kinetics of most all compounds on silica and degrade the separation. So, when it comes time to purify this type of reaction mixture what are your options?
- -Back-extract into a less polar solvent
- -Use reversed-phase flash chromatography
- -Evaporate the mix to dryness and reconstitute in a less polar solvent
- -Add a sorbent, mix, and evaporate to dryness
Since solvents such as DMF, DMSO, and NMP have broad solubility what would you choose as a back-extract solvent, hexane? Not likely to be effective as few compounds are solubilized by hexane or related hydrocarbon solvents.
Reversed-phase absolutely makes sense and in previous posts I have discussed direct DMF and DMSO injections. However, if your compounds are water-sensitive or if you do not have reversed-phase flash columns then this avenue is not an option.
Evaporating to dryness and reconstituting in another solvent makes sense as does drying the reaction mixture solution onto an adsorbent for dry loading. However, the high boiling points of these three solvents make evaporation by conventional means quite problematic (Table 1).
| Solvent | BP °C |
| DMF | 153 |
| DMSO | 189 |
| NMP | 202 |
Evaporation, however, is where I focused my sample work-up efforts with a DMF solvated reaction mixture.
My reaction was simple – benzaldehyde (180 mg) + isatoic anhydride (277 mg) + benzyl amine (182 mg) + acetic acid (102 mg) all dissolved in 3 mL of DMF to make 3-benzyl-2-phenyl-2,3-dihydro-4(1H)-quinazolinone - using a Biotage® Initiator+ microwave in 15 minutes at 220 ºC, Figure 1.

Figure 1. Automated microwave assisted organic synthesizers (MAOS) such as the Biotage® Initiator+ can help expedite synthesis using high boiling solvents.
Purification with a direct liquid injection is a challenge with the amount of reaction mixture (741 mg) and volume of DMF (~3 mL) to be injected. So, to evaluate and compare sample prep options, I split the reaction into thirds with each portion containing ~247 mg.
TLC of my reaction mix indicated that a hexanes-ethyl acetate gradient should be sufficient. My flash system calculated I needed a 10 gram Biotage® SNAP Ultra cartridge in order to purify each 247 mg portion of the reaction mixture. Based on this information I set up three purification routes for comparison and to see which is the preferred purification route…
- -No work-up, direct liquid injection of 1/3 of reaction mix
- -Reaction mixture evaporation with re-dissolution in DCM followed by direct liquid injection
- -Reaction mixture evaporation onto 1 gram of silica followed by dry-load flash purification using a Samplet® cartridge.
For reaction mixture evaporations I used the new Biotage® V-10 Touch. For those unfamiliar with this instrument it is a self-contained, open-access, high-speed evaporation system requiring no external intervention other than method selection and placement of the vial containing sample on the vial holder. This system has shown to be very efficient at evaporating all types of solvents including high boilers such as DMF (there’s even an optimized evaporation method just for high boilers!), Figure 2.

Figure 2. The V-10 Touch rapid solvent evaporation system was used to evaporate the DMF used in the reaction.
The chromatography results show that with a direct injection of 1 mL of reaction mix the separation is ok but not ideal, Figure 3. The product is broad, fronting (an indication of volume overload), and has both leading and trailing un-resolved impurities.
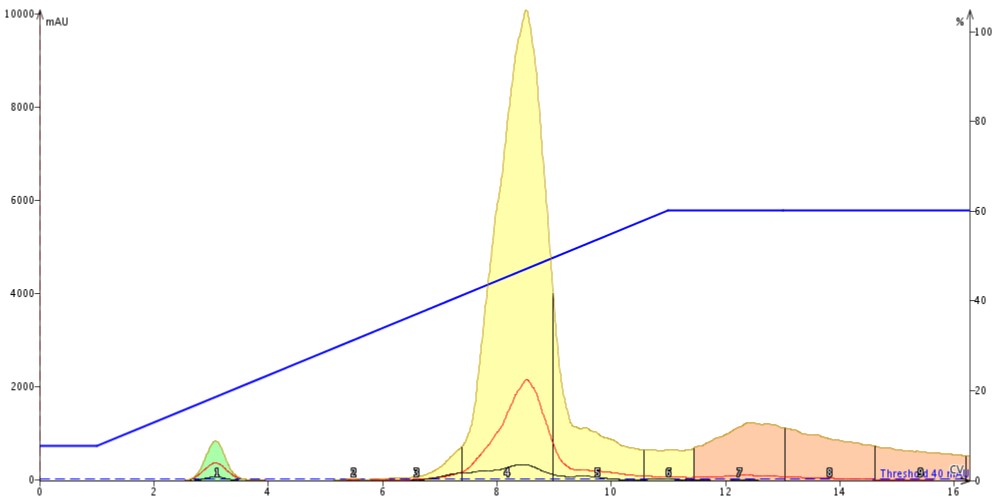 Figure 3. Normal-phase purification of 1 mL of reaction mixture containing 247 mg of reagents and product. The large solvent volume diminishes the amount of separation between the product and its nearest impurities.
Figure 3. Normal-phase purification of 1 mL of reaction mixture containing 247 mg of reagents and product. The large solvent volume diminishes the amount of separation between the product and its nearest impurities.
Evaporating 1 mL of the reaction mixture to dryness using the V-10 required only 5 minutes and 39 seconds. The blend of sample and silica, when evaporated, also required the same evaporation time.
The evaporated liquid reaction mixture was re-dissolved in 0.4 mL of DCM and injected onto a fresh 10 gram SNAP Ultra cartridge. The flash chromatography results show that by exchanging solvents from the polar, high-boiling DMF to the much less polar DCM that the purification is greatly improved, Figure 4. Fronting is no longer observed and there is more resolution between the product and its nearest neighbors.
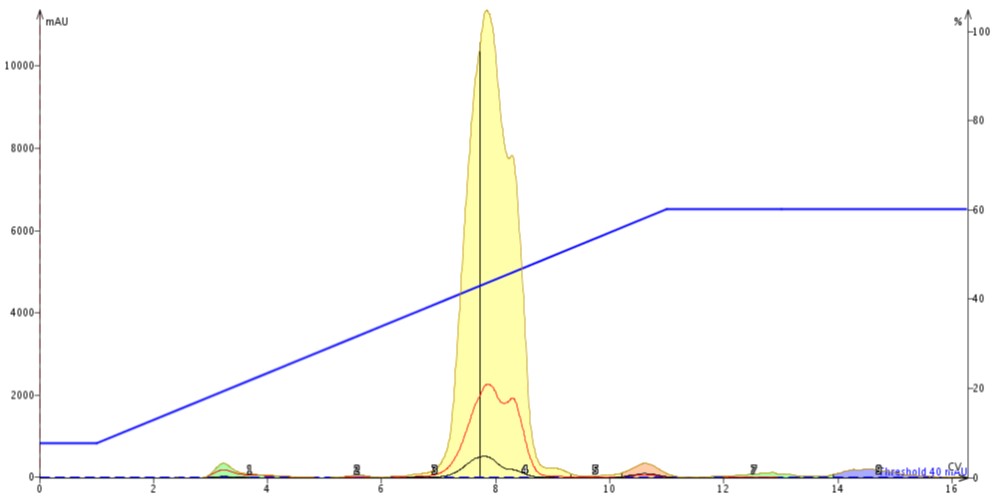
Figure 4. Normal-phase purification of 247 mg of reaction mix after drying on the V-10 Touch and re-dissolving in 0.4 mL of DCM. Evaporation of DMF and replacement with a smaller volume of DCM improves the purification.
Evaporating a reaction mixture onto a sorbent is an effective way to concentrate the reaction mix and prep it for purification. In this case I chose silica, 1 gram, to which I added the remaining third of the reaction mix. The liquid and silica were mixed with a spatula in a 20-mL scintillation vial and the contents evaporated to a free-flowing powder using the V-10 Touch. The dried powder was placed into an empty Samplet® cartridge which then was inserted into a new 10 gram SNAP Ultra cartridge. The results show a major improvement in separating the product from both leading and trailing impurities, Figure 5.
-3.jpg?width=998&name=3-comp-mix-dry-load%20(1)-3.jpg)
Figure 5. Normal-phase purification of the dried reaction mixture on silica (247 mg on 1 gram of silica). This technique provides the best separation pulling out a leading impurity and sharpening the main product peak as well.
So, the take-home message is that just because you need to use a polar, high boiling solvent for your synthesis you can still purify the reaction mix with normal-phase chromatography with a little bit of fast, 5-1/2 minute, sample prep with a high speed evaporator.
How have you dealt with purification of these types of reaction mixtures? Let us know if you have a proven process.
For more information on flash chromatography, please download our whitepaper - Success Flash Chromatography.

