May 5, 2023 2:00:00 PM
Homogenization and Extraction of PFAS from Solid Samples
By Christopher Mitchell

When it comes to environmental sample testing, there’s no shortage of the types of toxic substances you can detect utilizing liquid chromatography with tandem mass spectrometry (LC-MS/MS). At the time of injection in your LC-MS/MS system, they’ll all be in a liquid form, but they don’t always start off that way. Aquatic tissue, soil and sediments are common environmental matrices, which may contain toxic substances such as Per- and Polyfluorinated Substances (PFAS), within the solid sample. How do we get them from their natural solid state to a liquid? The answer is a technique known as homogenization and liquid-solid extraction. Let’s discuss what exactly this technique is and how we go about achieving this.
What is homogenization?
Homogenization is the process of breaking down a large complex sample into an individual, uniform mixture without incurring any degradation of your targe analytes. When a sample is homogenized, it makes it so much easier to analyze because you are testing a uniform mixture of all the sample components. Without homogenization, you cannot guarantee that your results are representative of the entire sample. Inconsistencies in your results may require the sample to be retested which can have a detrimental impact to the laboratory since you’ll always be working in conditions where sample and spike standards are a limited resource. To give you an idea of the variability that can exist in a solid sample, Figure 1 below shows different PFAS analytes that were detected within different locations of two samples, canned tuna, and frozen codfish. As you can see from the table, the concentration of PFAS analytes can be higher in certain portions of the sample, demonstrating that these toxic analytes may have preference for bioaccumulation in certain locations of the sample. Therefore, from a statistical standpoint we know that sample homogenization is key in screening solid matrices for toxic substances such as PFAS.
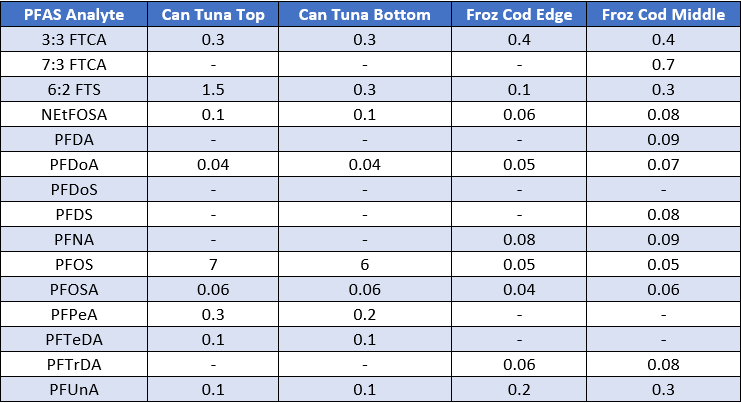 Figure 1: PFAS Detected in canned tuna and frozen codfish. All values in parts per billion (ppb).
Figure 1: PFAS Detected in canned tuna and frozen codfish. All values in parts per billion (ppb).
Homogenization Techniques
In laboratory settings, there definitely isn’t a shortage of homogenization techniques: bead mill, mortar and pestle and shearing are just to name a few. While any one of these is a suitable technique, we’re dealing with PFAS so contamination prevention will be key. With that being said, bead mill grinding is your ideal method to help minimize contamination. With this process it’s important to note that ceramic beads are the better option over glass beads since PFAS testing usually requires that the sample does not come into contact with glass prior to analysis. This is because glass is known to adsorb PFAS analytes onto its surface and therefore has been documented as being a source of possible contamination. For tissue samples you want to make sure that the samples are stored frozen in polypropylene tubes prior to use and handled in a semi-thawed state for more effective processing. With the nature of the application, you will not want to leave samples milling for an extended period of time as the collisions inside the container can significantly raise sample temperature and degrade some of the compounds. Short pauses, or dwell times, in the middle of the process can aid in countering this phenomenon and maintain low temperatures during homogenization. This makes systems like the Biotage® Lysera a perfect tool for quick, effective, and contaminant free homogenization of solid samples for PFAS analyses.
What is Liquid Solid Extraction?
Liquid Solid Extraction (LSE) is exactly what it sounds like. This technique utilizes a liquid solvent to extract certain analytes, such as PFAS, that are locked within a solid sample matrix. The benefit of using a bead mill homogenizer, such as the Biotage® Lysera, is that you can perform LSE of PFAS analytes in the same tube used for initial sample homogenization. This eliminates a transfer step and reduces any chances of sample loss during transfer.
Optimizing LSE Techniques
For successful LSE of your PFAS analytes from solid samples, it is important to evaluate both the solvents used for the extraction as well as the time needed for an effective extraction. Figure 2 below shows recoveries of LSE extracts from fish tissue samples for PFAS extracted internal standards (EIS) found in United States Environmental Protection Agency (EPA) method 1633. Data produced from Technique A uses 0.3% NH4OH in methanol as the extraction solvent on the Biotage® Lysera with total extraction time equating to ~15 minutes. Technique B also uses 0.3% NH4OH in methanol, however, an additional overnight extraction for a minimum of 16 hours was applied. The additional 16-hour shake was evaluated based on recommendation found in EPA method 1633. Results from technique A&B demonstrate that there was no benefit of adding a 16-hour extraction, and LSE could be effectively performed in about 15 minutes on the Biotage® Lysera. To test the extraction solvent, technique C utilized 0.05M KOH in methanol as performed in EPA method 1633. This resulted in similar analyte recoveries when compared to 0.3% NH4OH utilized in technique A&B, which suggests that both solvents could be suitable for LSE. Finally, technique D was the same as technique C, however, the LSE extract was pH adjusted with acetic acid prior to injection on the LC-MS/MS. This was done to simulate the final extract chemistry for EPA method 1633. A significant increase in recoveries were demonstrated in technique D. We believe it promotes superior reversed phase chromatographic separation of target analytes on the LCMSMS system leading to an improvement in analytical quantification. This demonstrates the importance of pH adjustments of extracts prior to analysis.
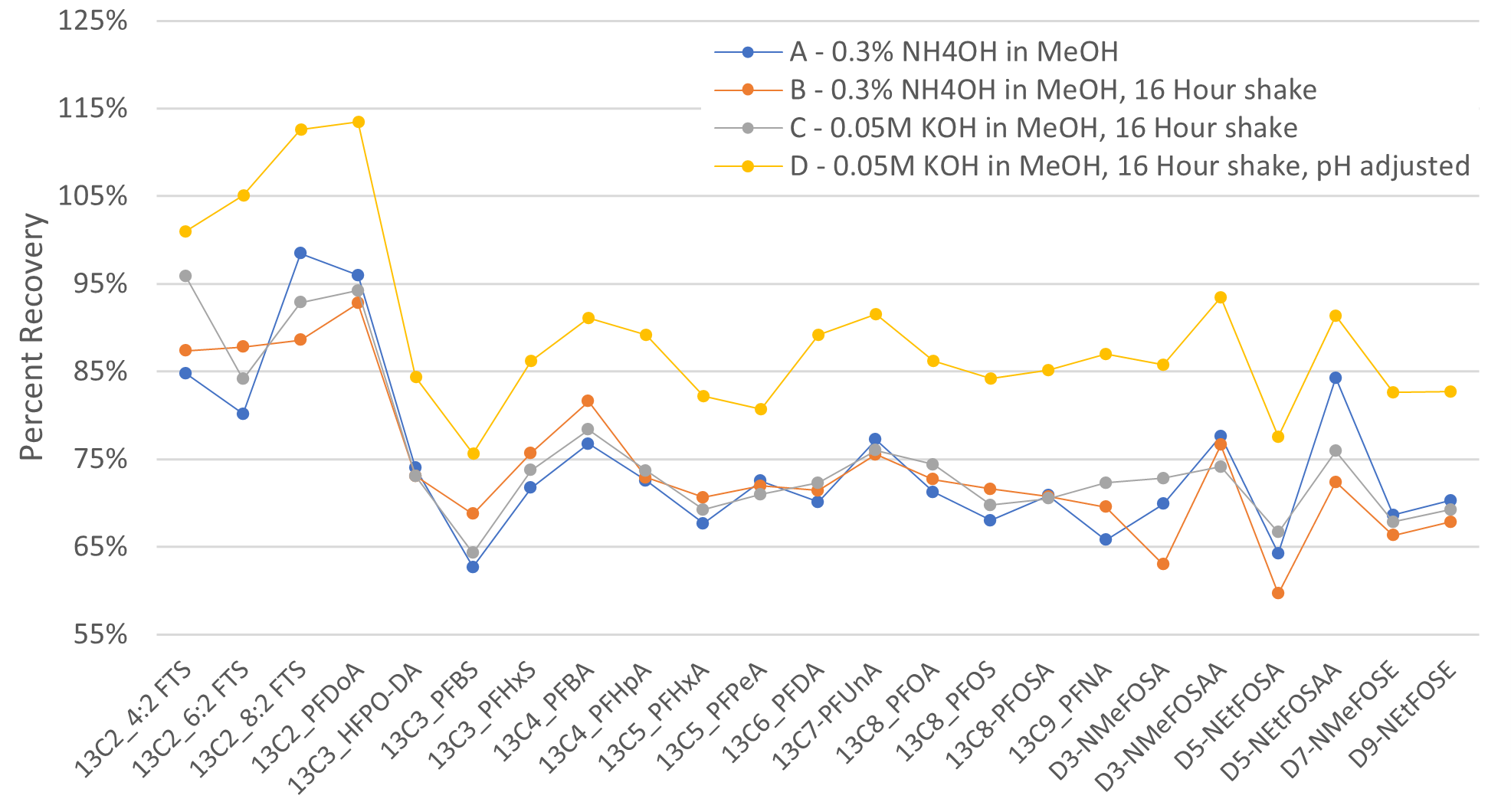 Figure 2: Evaluation of liquid solid extraction (LSE) extraction efficiency for extracted internal standards (EIS) found in EPA method 1633.
Figure 2: Evaluation of liquid solid extraction (LSE) extraction efficiency for extracted internal standards (EIS) found in EPA method 1633.
Conclusions
Detection of toxic compounds such as PFAS in solid matrices is extremely important for understanding how these compounds accumulate in our environment. Utilizing the Biotage® Lysera for homogenization ensures laboratories can take complex solid samples and produce homogenates that represent the sample in its entirety. Moreover, the Biotage® Lysera provides a mechanism for efficient liquid solid extraction from the same tube utilized for the homogenization. Therefore, when it comes to solid samples, the Biotage® Lysera is a great tool for any laboratory looking for an accurate and efficient way extract PFAS analytes.
Acknowledgement: A very special thank you to Lauren Dreyling from the Indiana Department of Health for helping us develop a protocol to homogenize and extract PFAS from fish tissue samples!
Published: May 5, 2023 2:00:00 PM

