Jan 23, 2023 2:05:48 PM
Are reversed-phase flash chromatography columns designed for aqueous solvents necessary?
By Bob Bickler

When it comes to the purification of polar, water-soluble compounds reversed-phase chromatography is the most commonly used approach. However, because of strong stationary phase – mobile phase repulsion forces, the use of highly aqueous (90-100% water) solvent systems has been shown to provide less retention than needed. This issue has led to the development of “aqueous compatible” reversed-phase media.
In this post I explore if these types of phases are actually needed by looking at the separation of some very polar and low log P compounds using a “traditional” C18-bonded silica.
Oil and water do not mix, as you know, and that is the situation with reversed-phase. This "phase separation" is the entire premise on which the reversed-phase chromatography is based – partitioning compounds between a polar solvent and an immiscible non-polar sorbent. However, because oil and water are immiscible strong repulsion forces are created by highly aqueous solvents. The repulsion forces cause reversed-phase chains to “collapse” as they become less miscible in the polar solvent environment. The stationary phase collapse reduces the available surface area for partitioning and compounds end up not retaining well and elute much earlier than expected. For many polar organic molecules, mobile phases consisting of 90% to 100% water are required to force the compounds to partition into the stationary phase. However, this can be problematic as the lipophilic bonded phase, e.g. C18, is repelled by the hydrophilic aqueous solvent. This repulsion minimizes the available surface area for the compounds’ to stick to the bonded phase. The issue relates to the wettability of the stationary phase which is quite wax-like.
To alleviate this issue some column/cartridge suppliers have developed C18 bonded phases designed to be compatible with very high water content mobile phases – at premium pricing. My research suggests that standard C18 cartridges, when properly conditioned, can be used for many of the same separations.
One of the keys to successful reversed-phase flash chromatography is pre-wetting the bonded phase, most often C18. Because reversed-phase flash cartridges are shipped dry they must be wetted with organic solvent first, typically either 100% methanol or 100% acetonitrile. After the initial wetting, the mobile phase polarity is gradually increased to 50% aqueous and then finally to your initial gradient conditions. I tend to do this in discreet steps - 100% organic for 5 CV, 50/50 for 5 CV, 10/90 for 5 CV, and finally 100% water for 5 CV (if I need to go below 10% organic solvent), Table 1.
| Solvent | Column volumes |
| 100% Acetonitrile or methanol | 5 |
| Acetonitrile or methanol with water (1:1) | 5 |
| Acetonitrile or methanol + water (10:90) | 5 |
| 100% Water | 5 |
As an example of a standard C18 cartridge's utility in a very aqueous environment, I used a Biotage® SNAP Ultra C18 with a linear gradient method starting in 100% water to separate a mix of commercially available food colors in water. If your crude sample contains ionizable/ionic organic species then the use of a volatile buffer should be employed to stabilize compound ionization. Buffers including ammonium formate, ammonium acetate, and ammonium carbonate are relatively volatile and are recommended for reversed-phase flash methods. Sometimes, though, pH adjustment with a volatile acid or base is needed to shift the compound’s equilibrium and increase its hydrophobicity.
Food colors contain sulfonic acid, carboxylic acid, and/or quaternary amine groups, Figure 1, which creates a need to use a buffer in the mobile phase. Chromatographic results with and without a buffer are shown in Figure 2.
Without buffer the ionic compounds are less retained (tartrazine elutes at the solvent front) and show what is known as "fronting". Fronting is a chromatographic phenomena typically related to ionized compounds not being in a stable ionic state. However, with an added buffer the dyes are retained longer and elute with a more compact Gaussian peak shape.
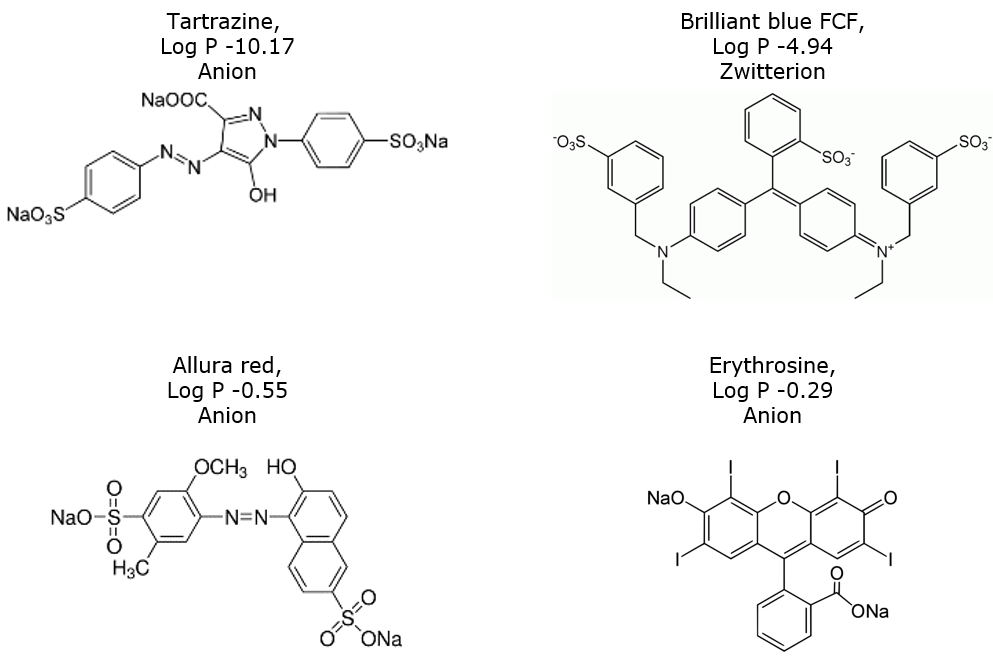
Figure 1. Four common ionic food colors
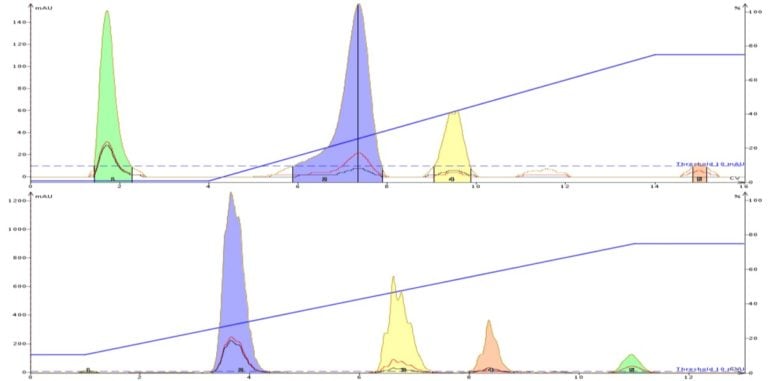
Figure 2. The benefits of adding a buffer to your solvent for the purification of ionizable compounds is seen here.
The top chromatogram is unbuffered resulting in the first dye (tartrazine) eluting early with little retention in 100% water. The other dyes (Allura red, Brilliant blue, erythrosine) show fronting and broadening (indicates they are existing in various ionic states). Adding a buffer reduces ionization and improves compound hydrophobicity providing for increased retention, compact elution bands, and the use of more organic solvent (gradient starts with 10% methanol rather than 0%).
But what if your compounds are not ionized in solution? Well, standard C18 cartridges, sometimes with buffers, can be useful there as well. The addition of buffers increases the solvent polarity and changes the mass transfer kinetics by forcing polar molecules to reside longer in the stationary phase. In Figure 3 I show an example of this with the separation of two compounds, uracil and caffeine dissolved in DMSO. Uracil is so polar it is often used as a void volume marker in reversed-phase HPLC column testing because of its lack of retention. However, with the addition of some buffer, a separation between uracil and DMSO is achieved and caffeine's peak shape improves as well.
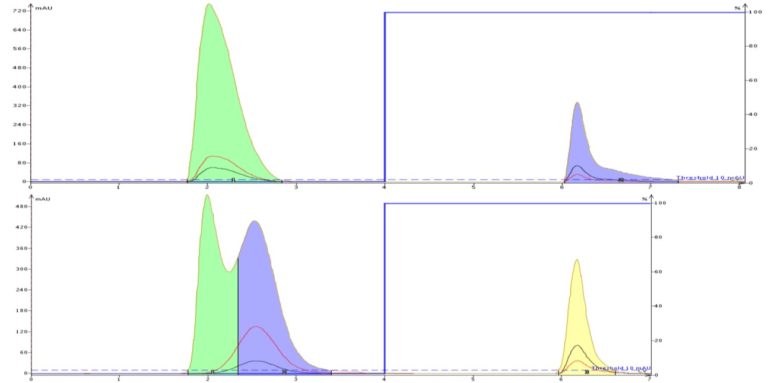
Figure 3. Non-ionic polar compounds also can benefit from buffers which make the solvent more polar. In the top chromatogram, uracil co-elutes with the injection solvent, DMSO, while caffeine is retained and shows significant tailing. The tailing extended the run and required extra solvent to finish. When buffer was added, uracil's retention improved enough to be fractionated separately from DMSO while caffeine eluted in a much tighter band reducing the amount of solvent needed for the purification.
So, while a specially designed C18 for highly aqueous mobile phases will likely work I believe a standard C18, when used properly, is capable of purifying very polar and ionizable compounds in highly aqueous solvents.
Have you used the aqueous compatible cartridges? Do they work better with your compounds?
If you are interested in learning more about flash chromatography, please download our white paper Successful Flash Chromatography.
Published: Jan 23, 2023 2:05:48 PM

